Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
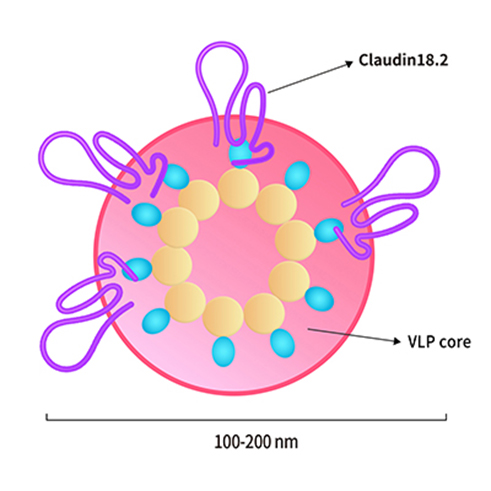
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the treatment of hematological malignancies, but solid tumors continue to pose significant challenges. Now, in the field of CAR T cell therapy, the development of solid tumors related therapies has been the direction of urgent breakthrough. Targeting Claudin18.2 in solid tumors has led to its extensive placement in the field of CAR T cell immunotherapy research.
For example, on November 15, 2021, Carsgen therapeutics announced that CT041, the world's first Claudin18.2 CAR-T candidate, has been granted PRIME status by the European Medicines Agency (EMA) for the treatment of gastric/gastroesophageal junction cancer. It is also worth mentioning that this is the world's first solid tumor CAR-T product selected for PRIME program. On January 20, 2022, Innovent Biologics registered a phase I clinical trial (IBI345) of Claudin 18.2-targeted CAR-T therapy on Clinical Trials. gov, which plans to enroll 30 patients with claudin 18.2-positive solid tumors. Breakthrough in solid tumor with CAR T cell therapy is on the way.
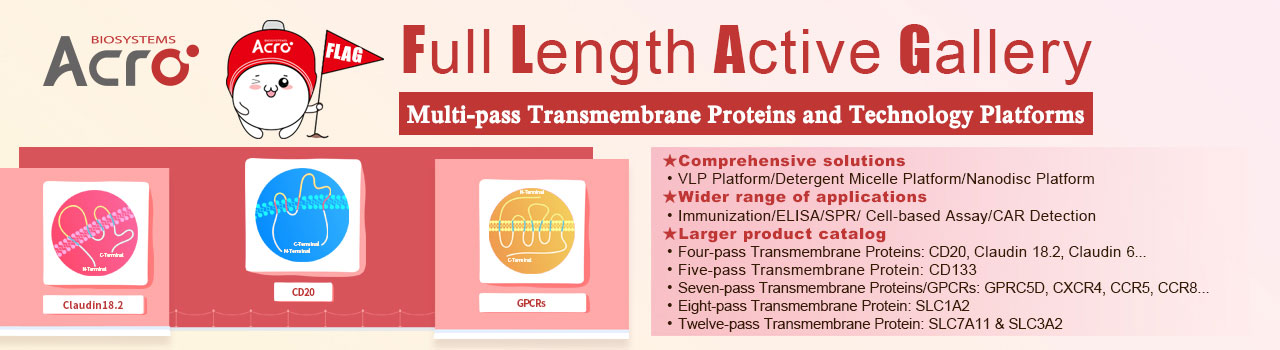
Claudin18.2-VLP(Cat. No. CL2-HF218)for Claudin18.2 CAR detection
ACROBiosystems has set up VLP(virus like particles)technology platform based on the HEK293 expression system to express full-length Claudin18.2 with native conformation on the surface of viroid particles. Claudin18.2-VLP(Cat. No. CL2-HF218)carries a GFP tag at the C-terminus and recommended for anti-Claudin18.2 CAR detection.
Reagents:
• Human Claudin-18.2 Protein (ACROBiosystems, Cat. No. CL2-HF218)
• Negative control protein (ACROBiosystems, Cat. No. VLP-NF2P4)
• FACS buffer: 2% BSA in PBS, pH7.2-7.4
Samples:
• Anti-Claudin-18.2 CAR-293 cells
Brief Protocol:
1. Culture Anti-Claudin-18.2 CAR-293 cells in DMEM medium with 10% FBS in the CO2 incubator (at 37 ℃, 5% CO2 ) respectively.
2. Harvest the cells and wash the cells once by FACS buffer.
3. Count the cells number and the viability, aliquot up 2×105 live cells into each tube. (Note: the cell viability must ≥ 95%.)
4. Dilute Human Claudin-18.2 Protein (Cat. No. CL2-HF218) or negative control protein in FACS buffer to get the working solution, and then add 100 μL of the working solution into the tube with cell pellet. Mix well and incubate at 4℃ for 60 minutes.
5. Wash the cells 3 times by FACS buffer and resuspend the cell pellet in 200 μL PBS per sample.
6. Transfer the cell suspension into flow tube and detect the cells by Flow cytometry.
7. Analyze the result data using FCS Express 6Plus and GraphPad Prism 5 software.
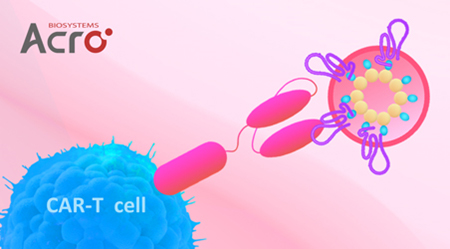
Results:
Human Claudin-18.2-VLP protein (Cat. No. CL2-HF218) and the negative control protein (Cat. No. VLP-NF2P4) were combined to transfect Claudin-18.2-CAR-293 cells. The results showed that Claudin 18.2-VLP protein (Cat. No.CL2- HF218) can specifically bind to Claudin-18.2-CAR-293 cells.
This web search service is supported by Google Inc.