
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
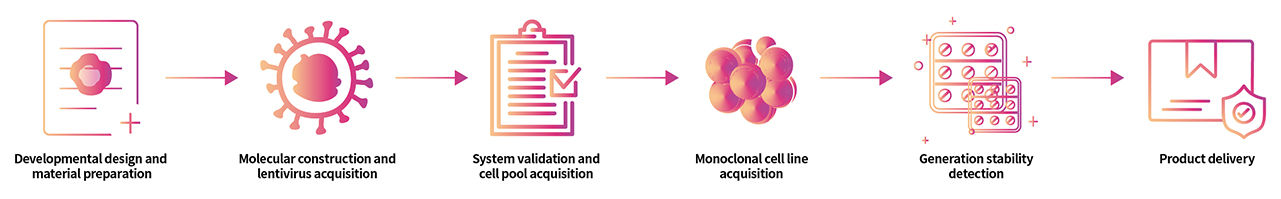
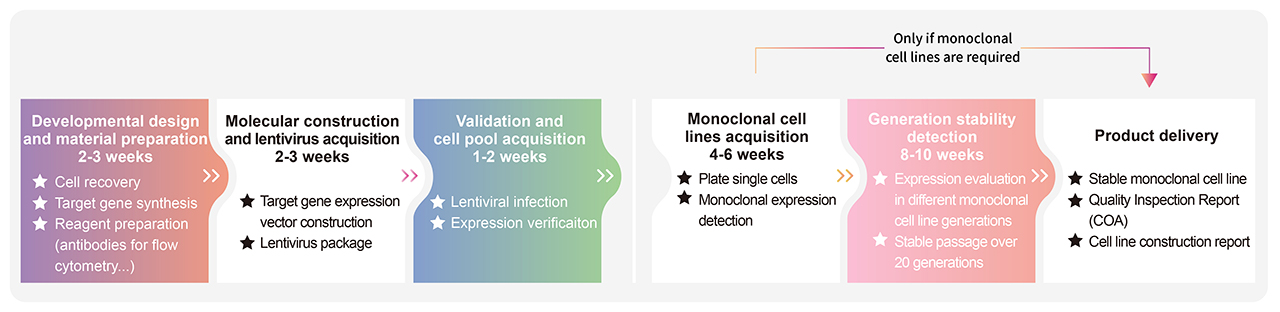
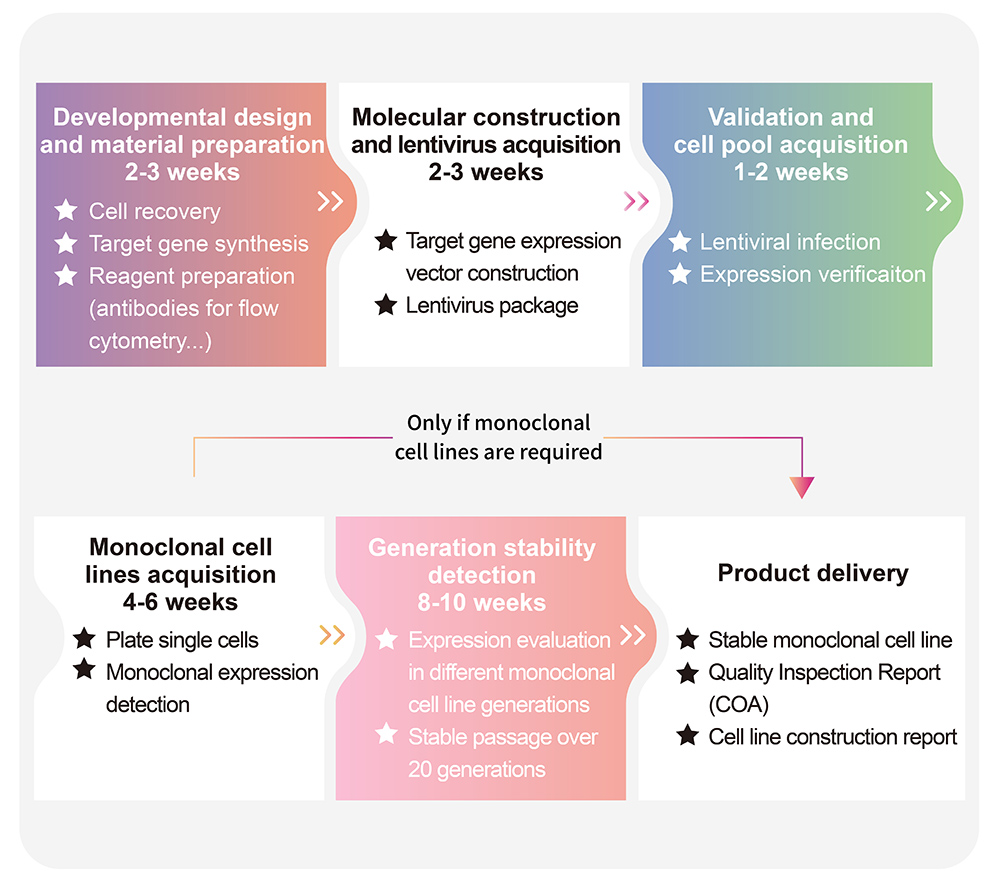
> Cell Line Customize Service
Throughout the developmental process of new therapeutics, a key factor is in the activity determination of antibody therapeutic drugs. In order to do so, transgenic cell lines are widely used due to its simplicity, speed, accuracy, non-biological safety, and animal substitution. Activity determination could be performed in various ways, including binding studies; however, in vitro evaluation is a critical part in drug development and quality control. In order to fully meet our customer's needs across their therapeutic development process, ACROBiosystsems now offers customization services for overexpression cell line to help you develop that perfect cell line!
>Gene function research
>Immunity/killing/proliferation, etc.
>Activity screening of antibody drugs (binding and blocking at the cellular level)
>Evaluation of the killing activity of CAR molecules
![]() Mature cell line development platform: Different overexpression and reporter gene cell line products are available.
Mature cell line development platform: Different overexpression and reporter gene cell line products are available.
![]() High-quality Deliverables: Strict quality control for strong reaction signals, wide detection windows, and long-term passaging stability without loss of expression.
High-quality Deliverables: Strict quality control for strong reaction signals, wide detection windows, and long-term passaging stability without loss of expression.
![]() Worry-free Service: Free sample testing before purchase backed by a professional technical support team and after-sales policy guarantee.
Worry-free Service: Free sample testing before purchase backed by a professional technical support team and after-sales policy guarantee.
![]() Regulatory documentation support: Quality inspection reports (CoA), cell line construction, cell traceability, and other documents required for CMC quality control release.
Regulatory documentation support: Quality inspection reports (CoA), cell line construction, cell traceability, and other documents required for CMC quality control release.
![]() One-stop customization: Only basic information required for development before delivery of cells & analytical reports. Regular reports and smooth communication is guaranteed throughout the service project.
One-stop customization: Only basic information required for development before delivery of cells & analytical reports. Regular reports and smooth communication is guaranteed throughout the service project.
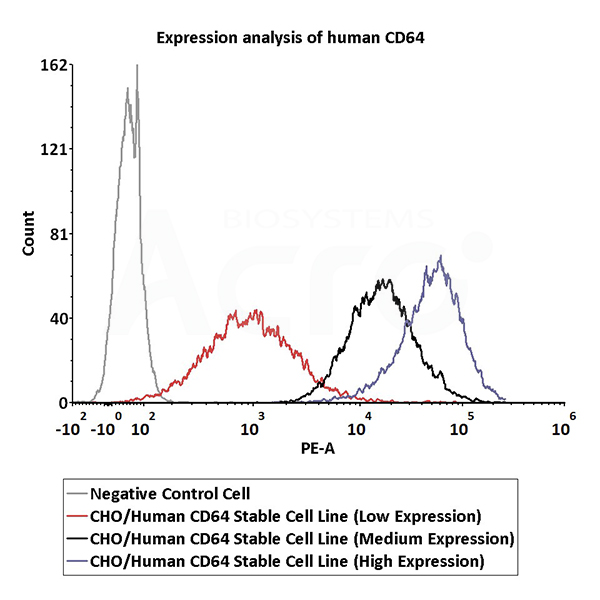
Expression analysis of human CD64 on CHO/Human CD64 Stable Cell Line by FACS. Cell surface staining using PE-labeled anti-human CD64 antibody was performed on CHO/Human CD64 Stable Cell Line with different expression levels: CHO/Human CD64 Stable Cell Line (Low Expression) (Cat. No. SCCHO-ATP062L); CHO/Human CD64 Stable Cell Line (Medium Expression) (Cat. No. SCCHO-ATP062M); CHO/Human CD64 Stable Cell Line (High Expression) (Cat. No. SCCHO-ATP062H).
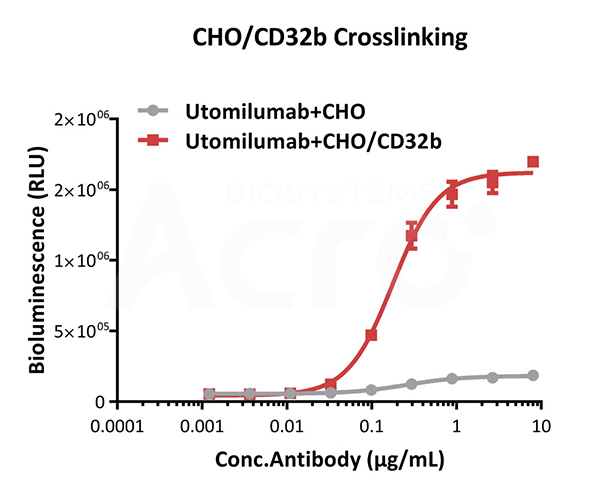
Bioactivity analysis of anti-human 4-1BB antibody through CHO/Human CD32b Stable Cell Line (Medium Expression) (Cat. No. SCCHO-ATP060M) crosslinking to test whether in a CD32b-dependent manner to strengthen the agonistic activity. The EC50 of anti-human 4-1BB antibody is approximately 0.18 μg/mL through CHO/Human CD32b Stable Cell Line (Medium Expression) crosslinking.
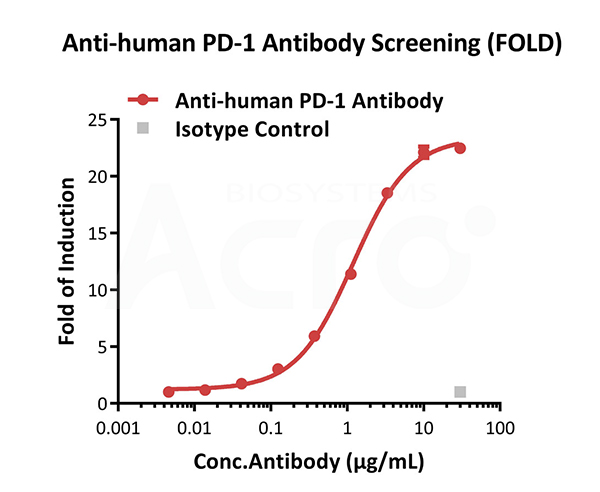
Blocking activity of anti-human PD-1 antibody (FOLD). This Raji/Human PD-L1 Stable Cell Line (Cat. No. SCRAJ-STT075) was incubated with serial dilutions of antibodies in the presence of reporter cells expressing human PD-1. The max induction fold was approximately 22.47.


This web search service is supported by Google Inc.








