 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Facteurs de croissance pour la culture cellulaire
Pour assister les fabricants et les développeurs de thérapies cellulaires en cours, ACROBiosystems a mis au point une large gamme de cytokines de haute qualité pour la culture in vitro de cellules immunitaires, de cellules souches, d'organoïdes et de divers autres types de cellules. Nous proposons également des cytokines de qualité ActiveMax® (à des fins de recherche uniquement), Premium(Pre-GMP) et GMP afin de mieux répondre aux besoins de différents stades de développement des médicaments et applications. Les trois catégories de cytokines sont fabriquées selon un processus de production similaire, ce qui permet une transition sans heurts entre les catégories. Ainsi, nous vous permettons d'effectuer une transition en douceur entre nos produits et d'accélérer votre recherche et développement.
| Qualité Premium(Pre-GMP) | Qualité GMP | |
|---|---|---|
| Application | Research and Development; Preclinical research, seamless transition into clinical phases | Designed to meet clinical phase requirements and bolster your IND application to various regulatory bodies. |
| Quality System | ISO 9001 /ISO 13485 Certified | ISO 9001 /ISO 13485 Certified (Development stage) GMP Quality Management System (Production stage) |
| Production | ISO certified facilities | GMP certified facilities |
| Transient or stable cell lines | Stable cell lines (Full inspection according to USP and ChP) | |
| Animal-origin free materials or BSE/TSE free | Animal-origin free materials or BSE/TSE free | |
| Pharmaceutical-grade materials | Pharmaceutical-grade materials | |
| Strict 2 grade series sterile filtration | Strict 2 grade series sterile filtration | |
| Class C+A room with manual aseptic filling (ISO5) | Class B+A cleanroom with automatic filling machine | |
| No additional virus clearance steps | 2 additional virus removal and inactivation steps (nanofiltration + low pH) | |
| Quality Control | Sterility / Mycoplasma testing | Sterility / Mycoplasma testing |
| Endotoxin control and detection | Endotoxin control and detection | |
| Validated key equipment and analytical instruments | Validated equipment /analytical instruments/analytical methods(Audit trail available) | |
| Residual DNA/HCP testing | Residual DNA/HCP testing | |
| Limited adventitious agent testing | Complete adventitious agent testing (virus testing and animal in vivo safety experiments) | |
| Documentation | Common regulatory support | Comprehensive regulatory support files |
| DMF filing (Few products) | DMF filing (All products) |
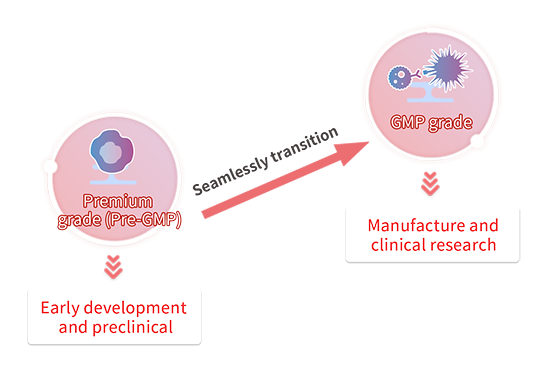
Passage de Premium(Pre-GMP) à GMP 
Les cytokines sont des matières premières essentielles utilisées dans la culture de cellules pour les médicaments de thérapie cellulaire et génique (CGT). Habituellement, au stade préclinique, la sécurité et la qualité des matières premières ne sont pas prioritaires. Dans ce cas, il est possible d'utiliser des produits destinés à la recherche uniquement. Toutefois, lorsque l'on passe à des étapes ultérieures du développement d'un médicament, telles que les phases CMC ou cliniques, il est nécessaire de remplacer les matières premières par des matériaux de qualité GMP afin de se conformer aux directives réglementaires. Pendant cette période de transition, une quantité importante d'énergie est consacrée à la réévaluation et à la validation de nouvelles matières premières.
Pour faciliter la transition entre le développement préclinique et le stade clinique, nous proposons plusieurs catégories de cytokines dont la bioactivité a été évaluée comme étant presque identique, ainsi que la documentation requise pour les produits GMP. Nous recommandons l'utilisation de nos matières premières de qualité Premium(Pre-GMP) au stade précoce du développement afin d'assurer une transition sans heurts vers nos matières premières de qualité GMP lors de l'entrée en phase CMC ou clinique et de minimiser le nombre d'études de réévaluation et de validation réalisées.
![]() Stérile
Stérile
![]() Sans composants d'origine animale
Sans composants d'origine animale
![]() Haute Pureté≥95%
Haute Pureté≥95%
![]() Haute Bioactivité Vérifiée par Test Cellulaires
Haute Bioactivité Vérifiée par Test Cellulaires
![]() Qualités Premium(Pre-GMP) et GMP disponibles
Qualités Premium(Pre-GMP) et GMP disponibles
![]() Faible endotoxine ≤0.1 EU/μg
Faible endotoxine ≤0.1 EU/μg
![]() Sans porteur
Sans porteur
![]() Semblable à la conformation naturelle et modifications
Semblable à la conformation naturelle et modifications
![]() constance entre les lots
constance entre les lots
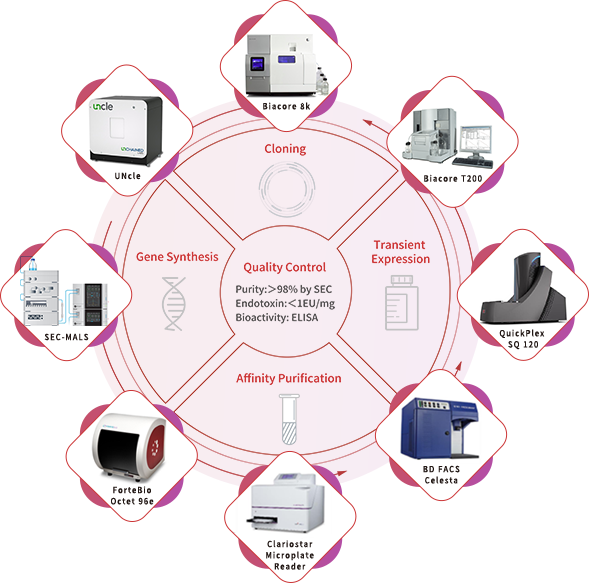
With a portfolio of over 5,000 recombinant proteins and an industry-leading, scale-up ready protein development platform, ACROBiosystems has accumulated over 10 years of experience in developing recombinant proteins. Using this platform, our custom GMP-grade protein services are designed to ensure that our proteins are both structurally designed and validated for cellular therapies manufacturing. We take care to adhere strictly to the GMP guidelines with our comprehensive quality management system and quality controls, providing you with high-quality raw materials without disrupting your development process. Our custom GMP-grade protein service is a one-stop service based on your needs to maximize your therapy’s success. We offer two different developmental processes: converting our non-GMP protein products to GMP or developing a custom GMP-grade protein product from scratch.

ActiveMax® Human G-CSF, Tag Free (Cat. No.GCF-H5214) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.
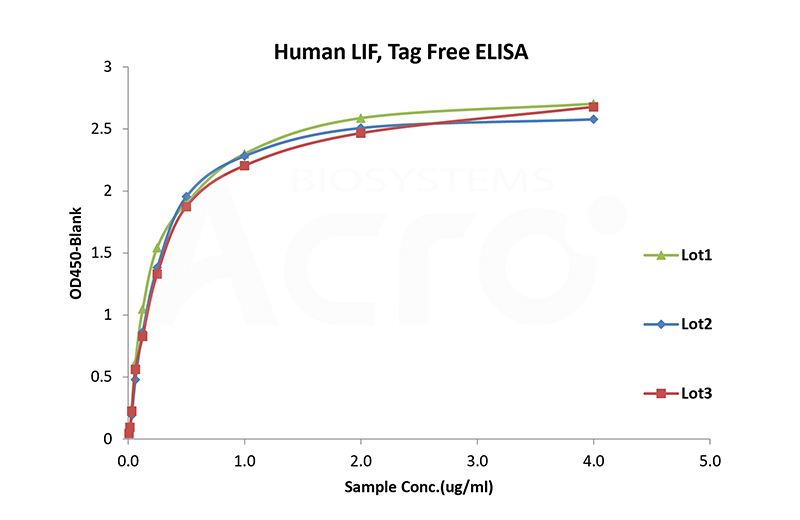
Bioactivity of three different lots of ActiveMax® Recombinant Human LIF (Cat. No. LIF-H521b) is verified by ELISA, and the result shows very high batch-to-batch consistency.
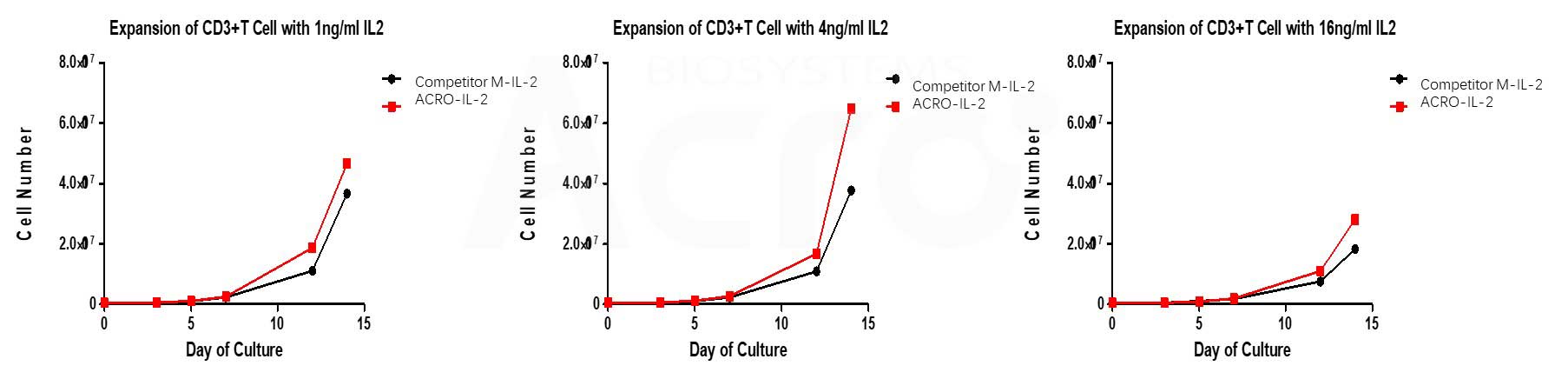
ACRO’s Human IL-2, premium grade (Cat. No.IL2-H5215) has higher bioactivity than that of other competitors when activates T cell with CD3/CD28 magnetic beads at different concentrations.
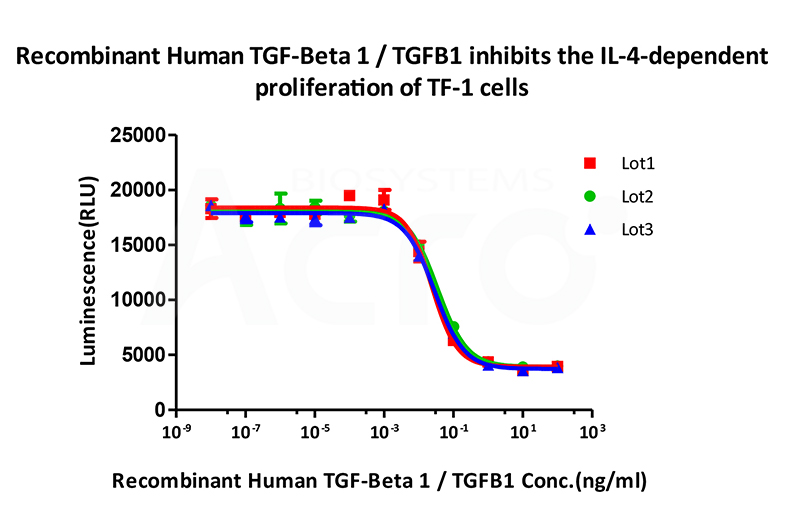
Bioactivity of three different lots of Recombinant Human TGF-Beta 1 TGFB1 (Cat. No. TG1-H4212) is verified by cell-based assay, and the result shows very high batch-to-batch consistency.
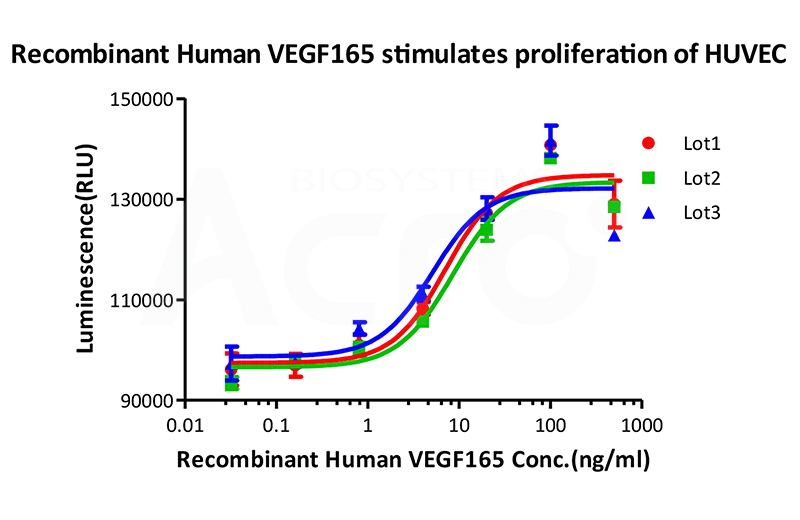
Bioactivity of three different lots of Recombinant Human VEGF165 (Cat. No. VE5-H4210) is verified by cell-based assay, and the result shows very high batch-to-batch consistency.
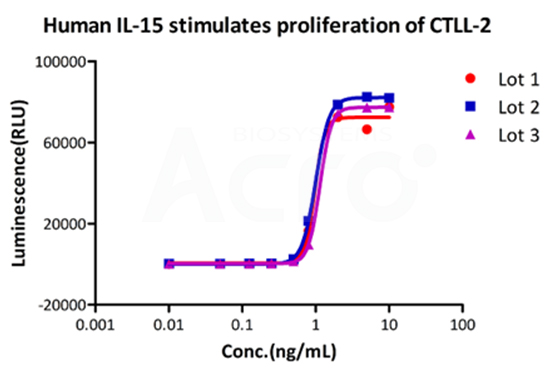
Recombinant Human IL-15 Protein (premium grade), designed for preclinical stage, has the same functional activity and performance as GMP Grade IL-15 (Cat. No.GMP-L15H13), which enables a seamless transition from preclinical development to clinical phases.
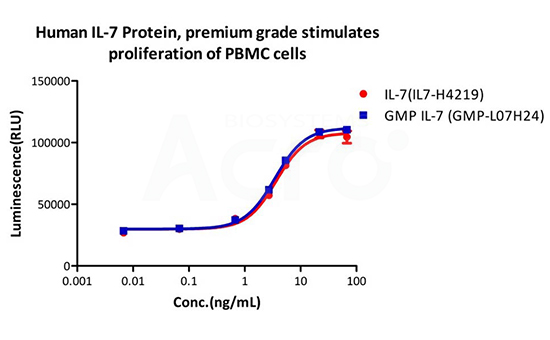
Human IL-7 Protein (premium grade) designed for preclinical stage, has the same functional activity and performance as GMP Grade IL-7 (Cat. No. GMP-L07H24), which enables a seamless transition from preclinical development to clinical phases.
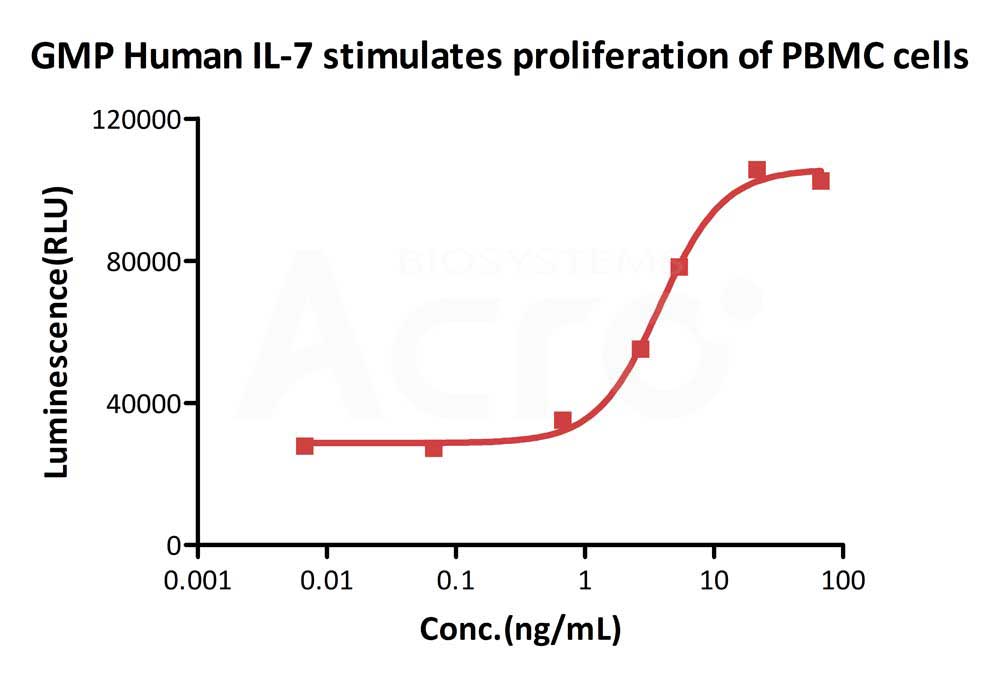
GMP Human IL-7 (Cat. No.GMP-L07H24) stimulates proliferation of PHA-P-activated human peripheral blood mononuclear cell (PBMC). The EC50 for this effect is 3.821 ng/mL, corresponding to a specific activity of > 1.0 ⅹ10^8 IU/mg, which is calibrated against human IL-7 WHO International Standard (NIBSC code: 90/530) (QC tested).
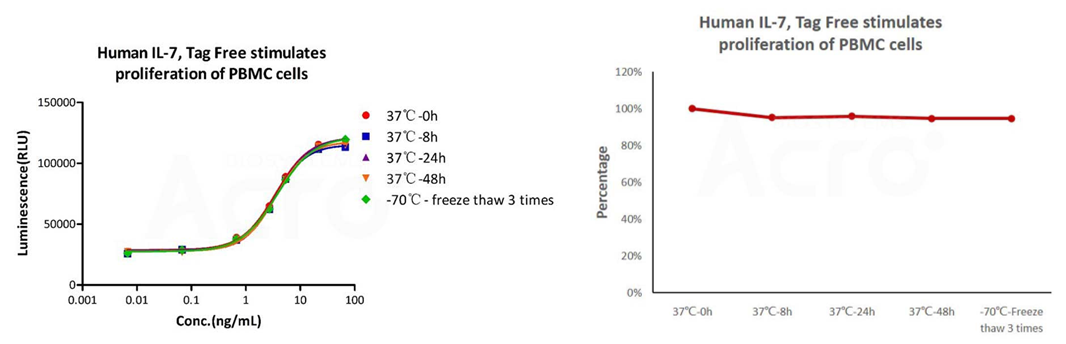
The cell-based assay shows that GMP Human IL-7 (Cat. No. GMP-L07H24) is stable at 37°C for 48 hours and after freezing and thawing 3 times.

Bioactivity of three different lots of GMP Human IL-15 (Cat. No.GMP-L15H13) verified by cell-based assay, and the result shows very high batch-to-batch consistency.
This web search service is supported by Google Inc.
