 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
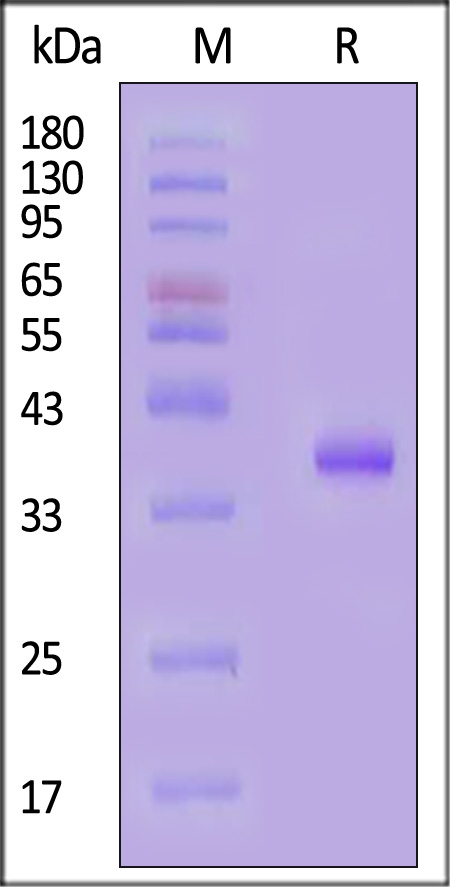
| IG1-H5263 | Human | Human IGF-I Protein, Fc Tag (MALS verified) |  |
|
|
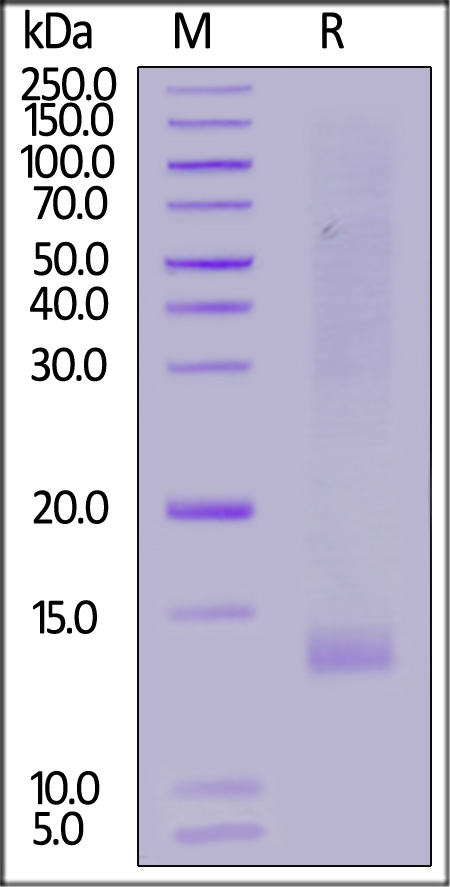
| IG1-H82Q6 | Human | Biotinylated Human IGF-I Protein, His,Avitag™ (SPR verified) |  |
|
|
| IG1-H5245 | Human | Human IGF-I Protein, His Tag (SPR verified) |  |
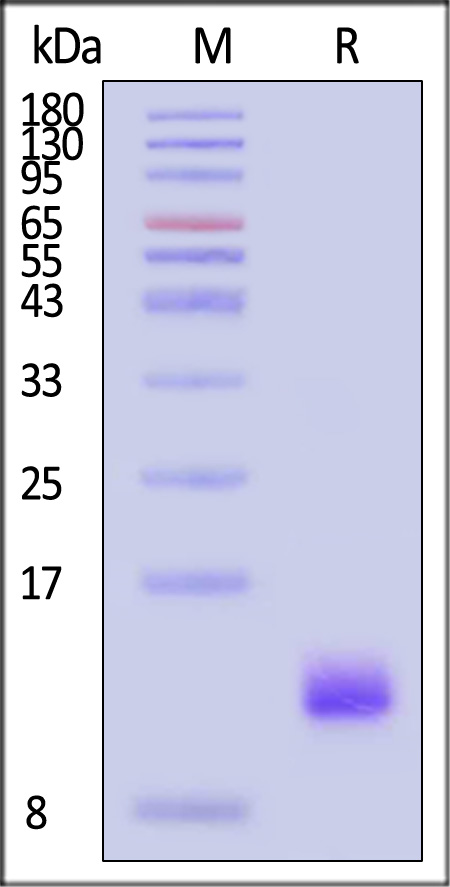
|
|
| IG1-M5253 | Mouse | Mouse IGF-I Protein, Fc Tag (MALS verified) |  |

|
|
| IG1-H82F7 | Human | Biotinylated Human IGF-I Protein, Avitag™,Fc Tag (MALS verified) |  |
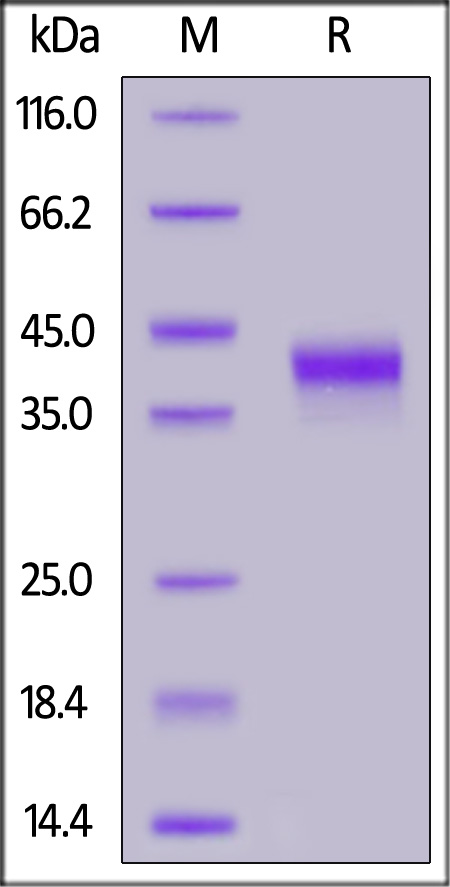
|
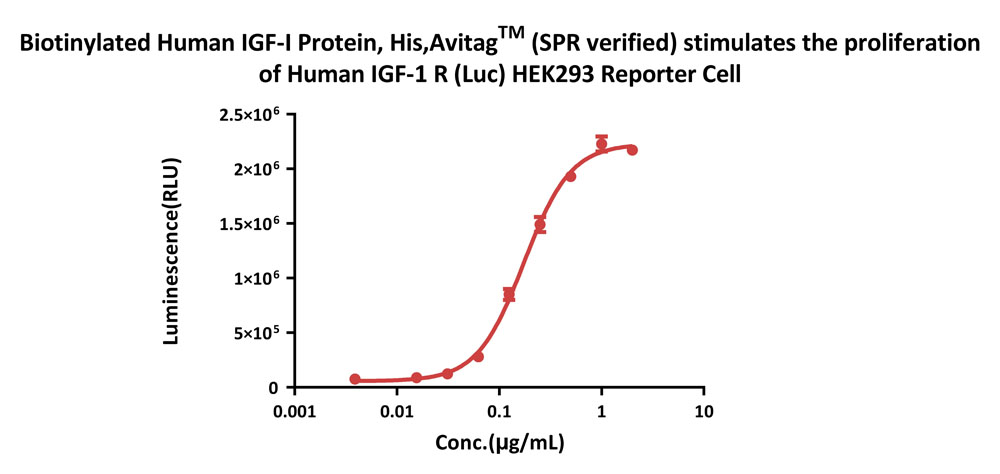
Biotinylated Human IGF-I Protein, His,Avitag™ (SPR verified) (Cat.No. IG1-H82Q6) stimulates the proliferation of Human IGF-1 R (Luc) HEK293 Reporter Cell. The EC50 for this effect is 0.1746 µg/mL. (Routinely tested)
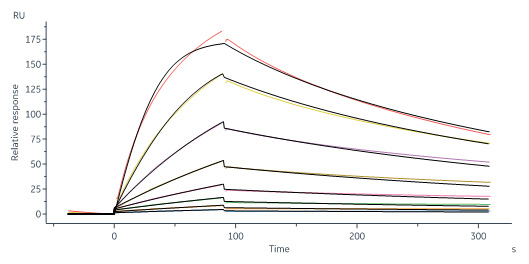
Human IGF-I R, His Tag (Cat. No. IGR-H5229) immobilized on CM5 Chip can bind Biotinylated Human IGF-I, His,Avitag (Cat. No. IG1-H82Q6) with an affinity constant of 68 nM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ACT-01 | ACT-01; OCS-05; BN-201; G-79 | Phase 2 Clinical | Promius Pharma | Keratosis, Actinic; Optic Neuritis; Demyelinating Diseases | Details |
| Xentuzumab | BI-836845 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Dusigitumab | MEDI-573 | Phase 2 Clinical | Medimmune Llc | Solid tumours; Neoplasms; Breast Neoplasms; Carcinoma, Hepatocellular | Details |
| ACT-01 | ACT-01; OCS-05; BN-201; G-79 | Phase 2 Clinical | Promius Pharma | Keratosis, Actinic; Optic Neuritis; Demyelinating Diseases | Details |
| Xentuzumab | BI-836845 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Dusigitumab | MEDI-573 | Phase 2 Clinical | Medimmune Llc | Solid tumours; Neoplasms; Breast Neoplasms; Carcinoma, Hepatocellular | Details |
This web search service is supported by Google Inc.
