 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| GMP-PDBH19 | Human | GMP Human PDGF-BB Protein |  |
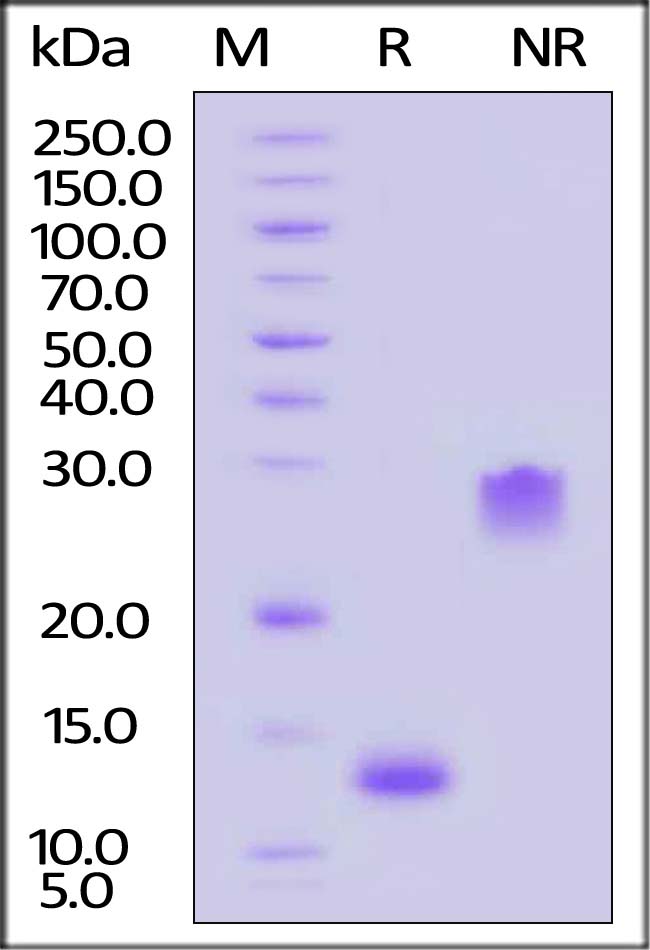
|
|
| PDB-H4112 | Human | Human PDGF-BB Protein, premium grade |  |
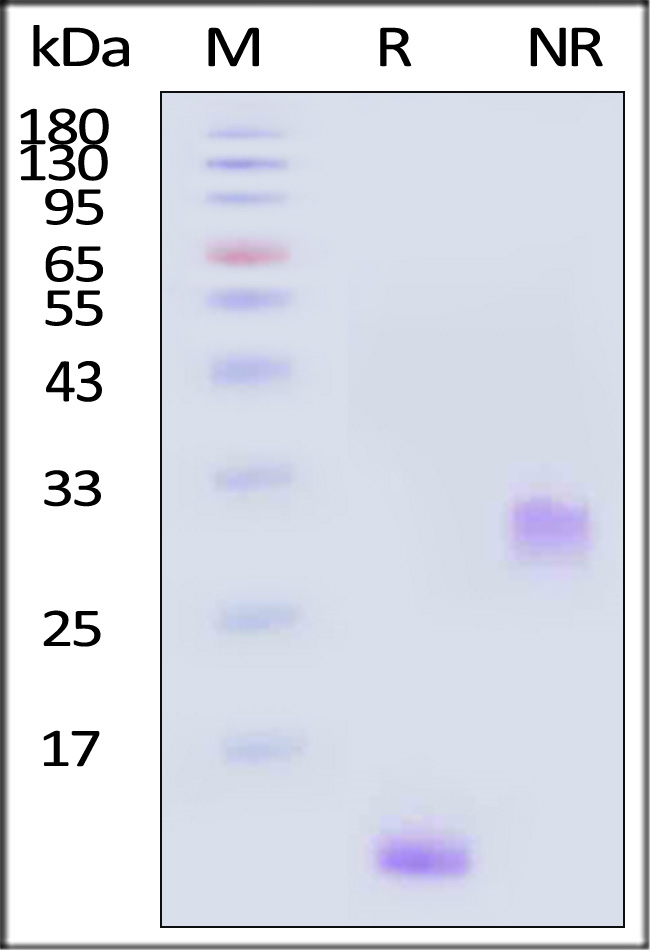
|
|
| PDB-H5127 | Human | Unconjugated Human PDGF-BB Protein, His,Avitag™ | 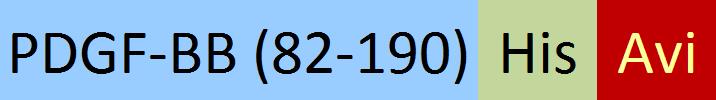 |
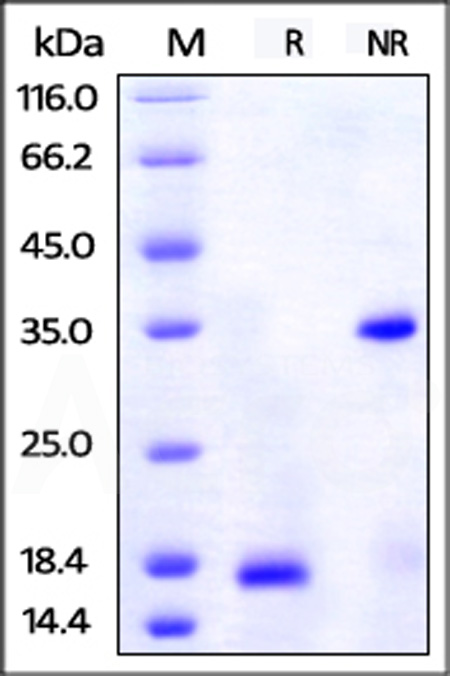
|
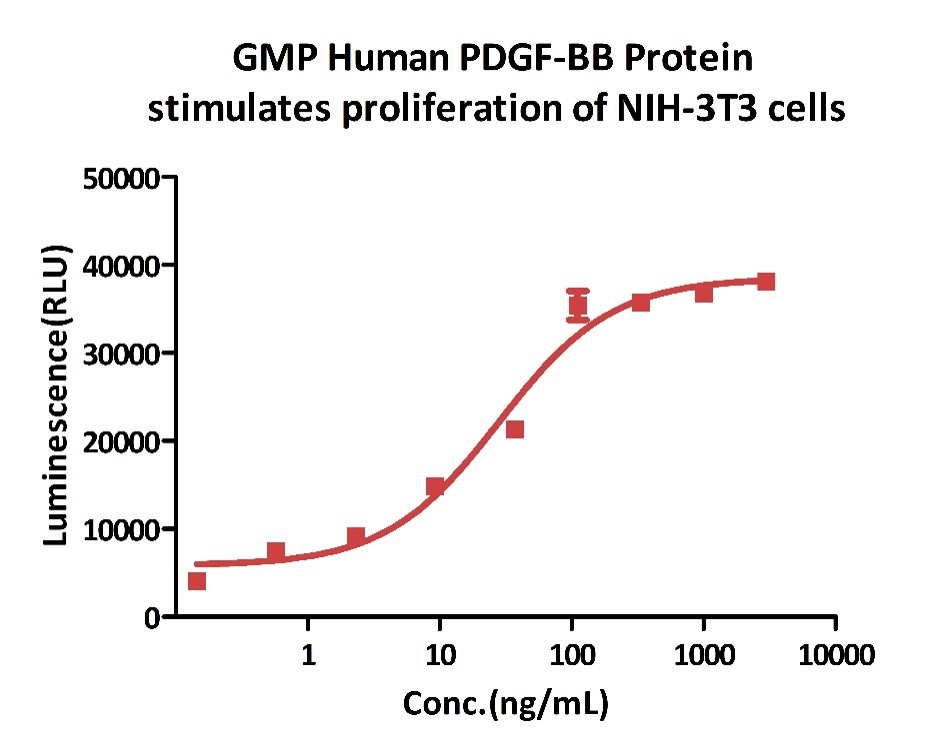
GMP Human PDGF-BB Protein (Cat. No. GMP-PDBH19) stimulates proliferation of NIH/3T3 cells. The specific activity of GMP Human PDGF-BB Protein is >5.00 x 10^5 IU/mg, which is calibrated against human PDGF-BB WHO International Standard (NIBSC code: 94/728) (QC tested).
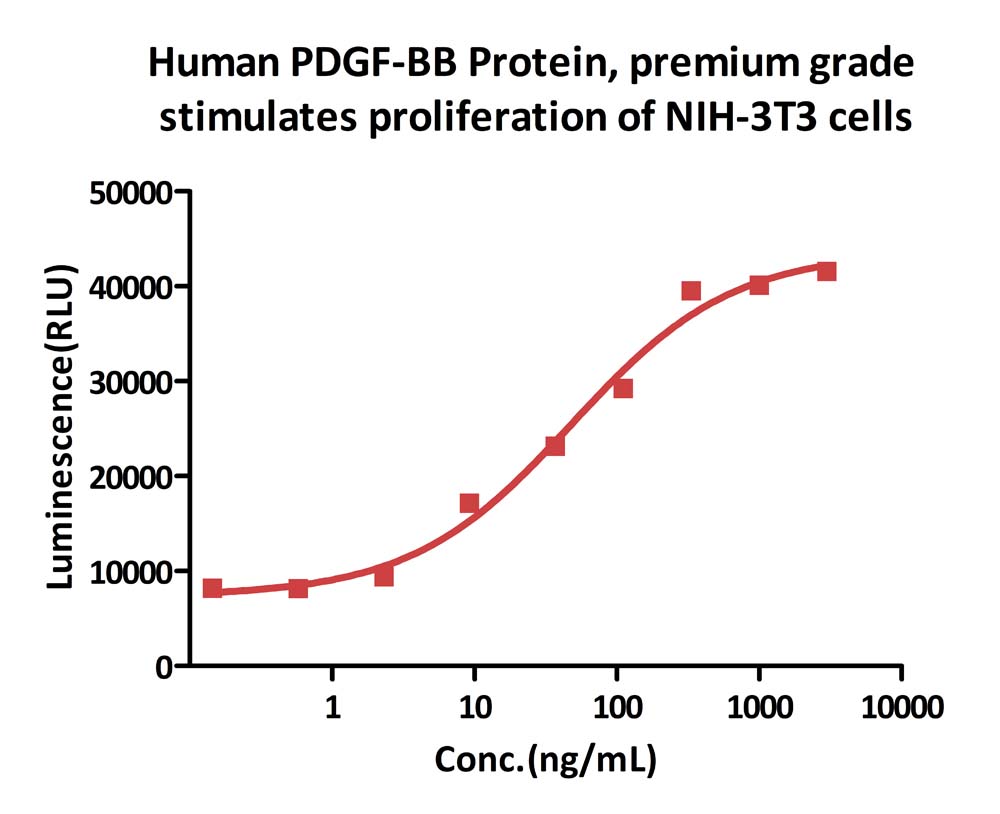
Human PDGF-BB, premium grade (Cat. No. PDB-H4112) stimulates proliferation of NIH/3T3 cells. The specific activity of Human PDGF-BB, premium grade is >5.00 x 10^5 IU/mg, which is calibrated against human PDGF-BB WHO International Standard (NIBSC code: 94/728) (QC tested).
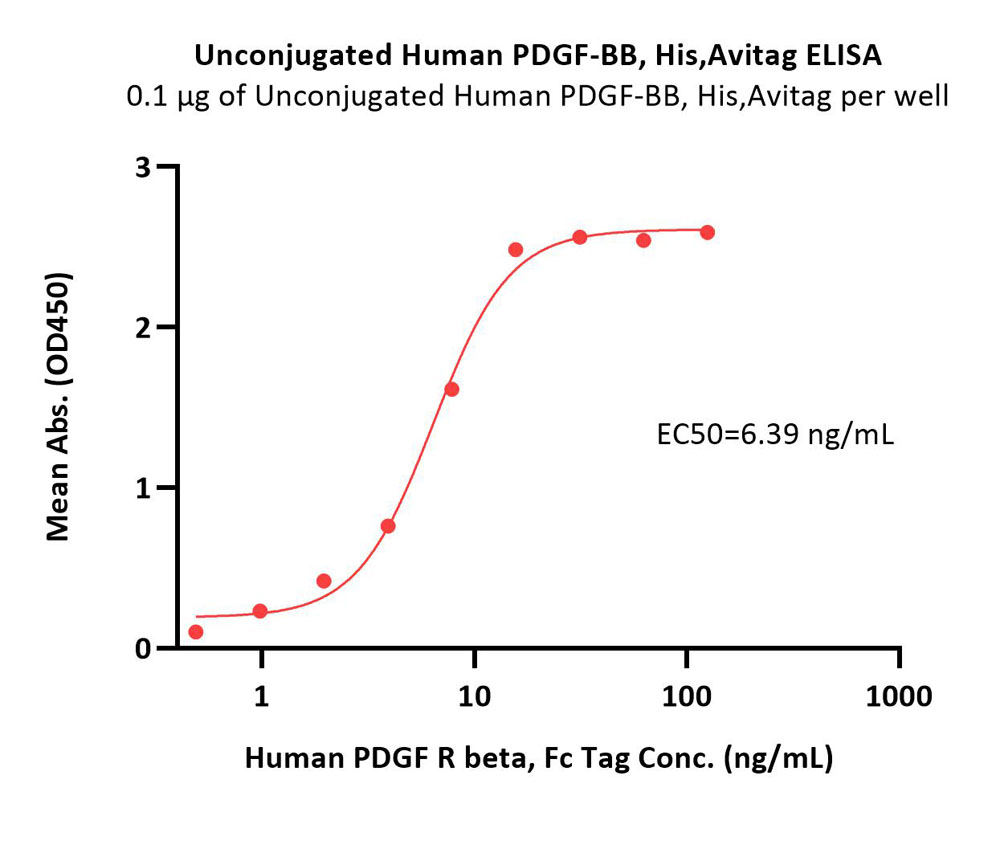
Immobilized Unconjugated Human PDGF-BB, His,Avitag (Cat. No. PDB-H5127) at 1 μg/mL (100 μL/well) can bind Human PDGF R beta, Fc Tag (Cat. No. PDB-H5259) with a linear range of 0.5-16 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| SHP-627 | FT-11; FT-001; SHP-627; FT-011 | Phase 2 Clinical | Shire Pharmaceuticals | Scleroderma, Systemic; Retinal Degeneration; Scleroderma, Diffuse | Details |
| Pegpleranib Sodium | X-01E; OAP-030; E-01AJ; DD50D2QKH1 (UNII code); E-10030 | Phase 2 Clinical | Archemix Corp, Osi Pharmaceutical | Macular Degeneration; von Hippel-Lindau Disease | Details |
| HL-217 | HL-217; KR-31831 | Phase 1 Clinical | Korea Research Institute Of Chemical Technology | Macular Degeneration | Details |
| REGN-13335 | REGN13335; REGN-13335 | Phase 1 Clinical | Details | ||
| SHP-627 | FT-11; FT-001; SHP-627; FT-011 | Phase 2 Clinical | Shire Pharmaceuticals | Scleroderma, Systemic; Retinal Degeneration; Scleroderma, Diffuse | Details |
| Pegpleranib Sodium | X-01E; OAP-030; E-01AJ; DD50D2QKH1 (UNII code); E-10030 | Phase 2 Clinical | Archemix Corp, Osi Pharmaceutical | Macular Degeneration; von Hippel-Lindau Disease | Details |
| HL-217 | HL-217; KR-31831 | Phase 1 Clinical | Korea Research Institute Of Chemical Technology | Macular Degeneration | Details |
| REGN-13335 | REGN13335; REGN-13335 | Phase 1 Clinical | Details |
This web search service is supported by Google Inc.
