 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| RAS-SP010 | Mouse | Mouse IL-4 ELISPOT Kit | |||
| GMP-L04H26 | Human | GMP Human IL-4 Protein |  |

|
|
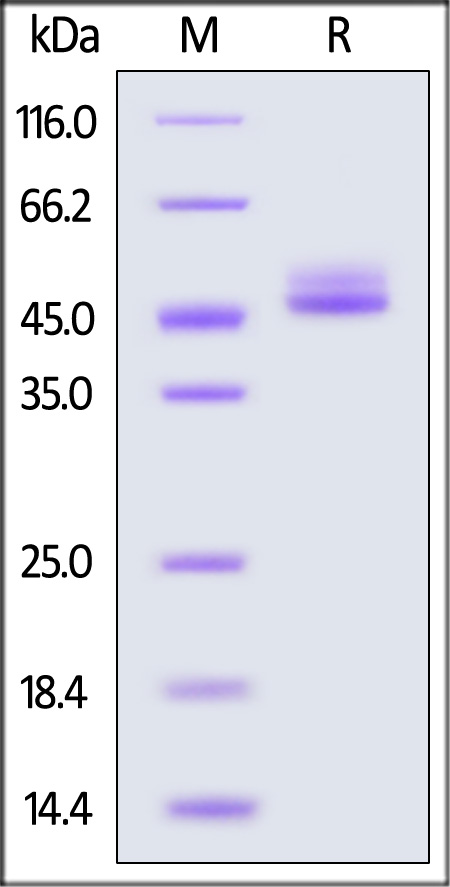
| IL4-H5253 | Human | Human IL-4 Protein, Fc Tag (MALS verified) |  |
|
|
| CRS-B003 | Human | ClinMax™ Human IL-4 ELISA Kit | |||
| EP-132 | Human | IL-4 [Biotinylated] : IL-4Rα Inhibitor Screening ELISA Kit | |||
| CRS-A004 | Human | resDetect™ Human Interleukin-4 (IL-4) ELISA Kit (Residue Testing) | |||
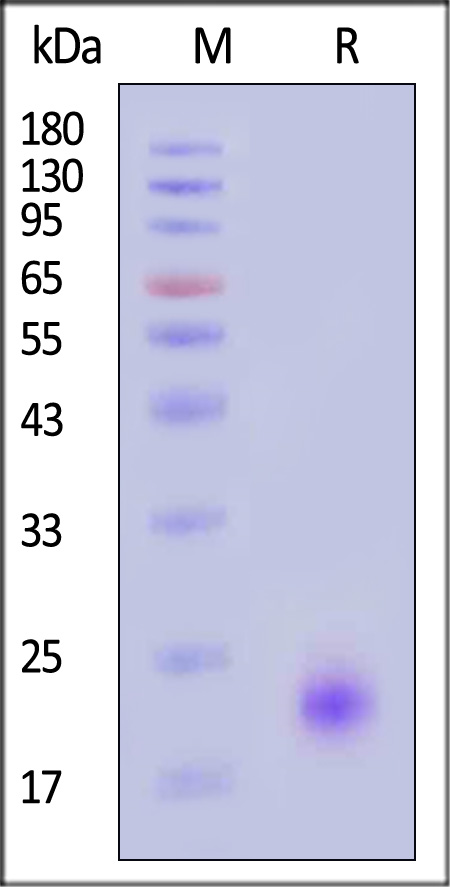
| IL4-H52H9 | Human | Human IL-4 Protein, His Tag (MALS verified) |  |
|
|
| IL4-C5259 | Cynomolgus | Cynomolgus IL-4 Protein, Fc Tag (MALS verified) |  |

|
|
| IL4-M52H5 | Mouse | Mouse IL-4 Protein, His Tag |  |
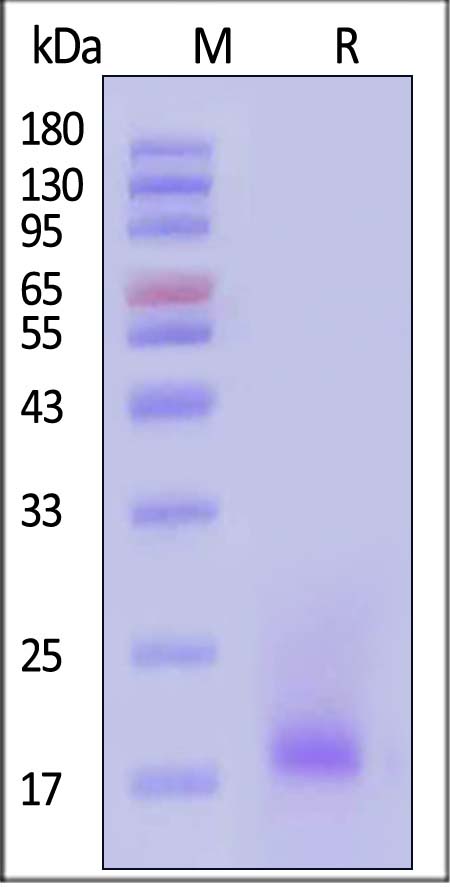
|
|
| IL4-H82E0 | Human | Biotinylated Human IL-4 Protein, Avitag™,His Tag (MALS verified) |  |
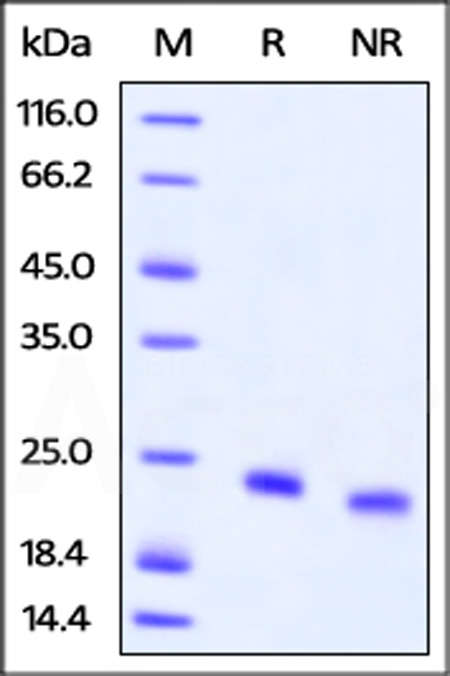
|
|
| IL4-H4218 | Human | Human IL-4 Protein, premium grade |  |
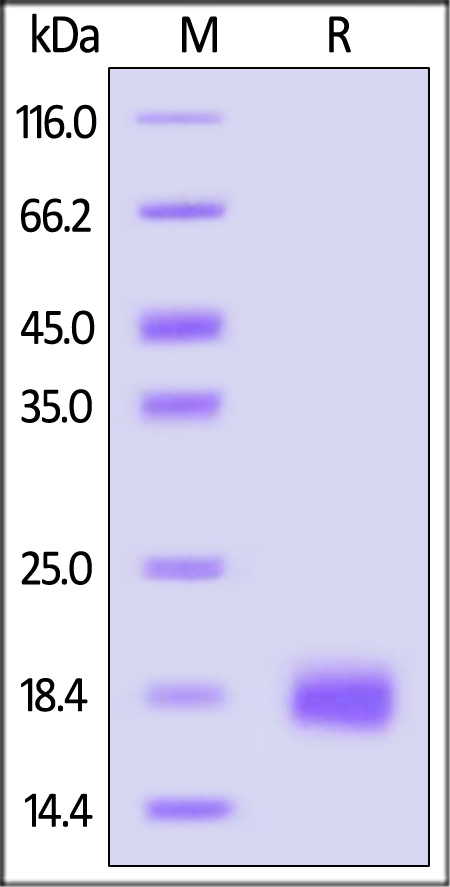
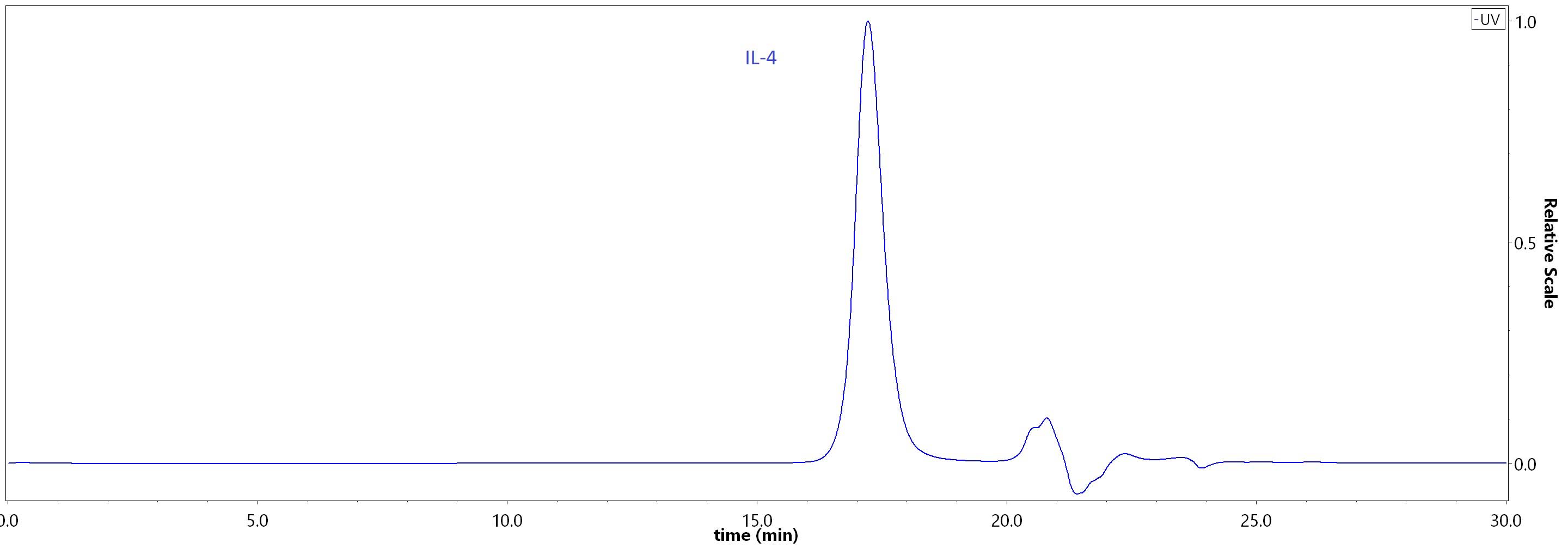
|
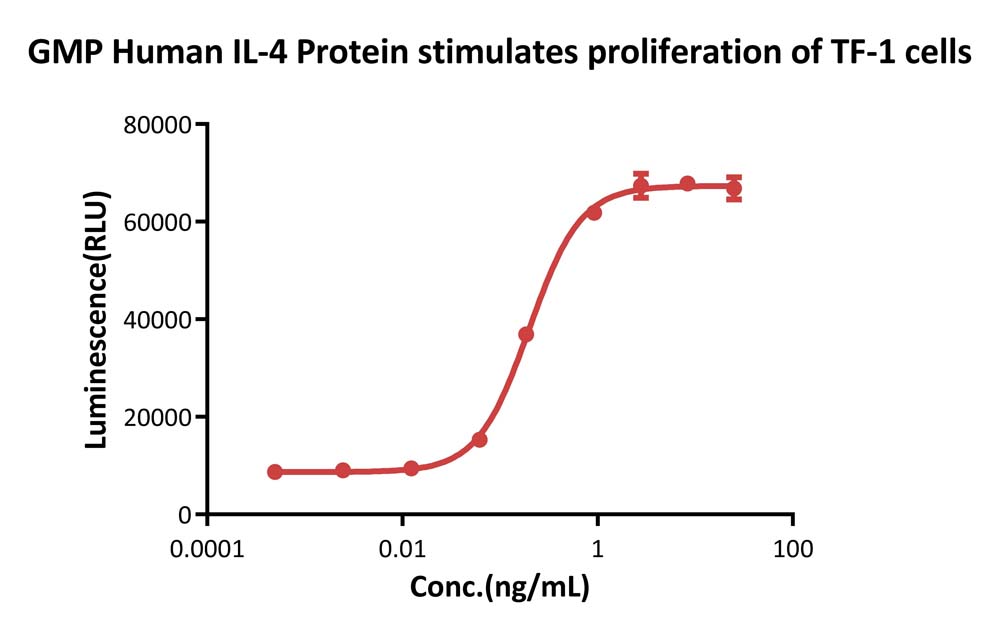
GMP Human IL-4 Protein (Cat. No. GMP-L04H26) stimulates proliferation of TF-1 human erythroleukemic cell line. The specific activity of GMP Human IL-4 Protein is > 1.20×10^7 IU/mg, which is calibrated against human IL-4 WHO International Standard (NIBSC code: 88/656) (QC tested).
![IL-4 [Biotinylated] : IL-4Rα Inhibitor Screening ELISA KitIL-4 [Biotinylated] : IL-4Rα Inhibitor Screening ELISA Kit (Cat. No. EP-132) ELISA bioactivity](/static/main/products/images/elisa/EP-132-E1.jpg)
INHIBITION OF IL-4 [BIOTINYLATED]: IL-4 R ALPHA BINDING BY Human IL-4
Serial dilutions of Human IL-4(Catalog # EP132-C03) (1:1 serial dilution, from 5 μg/mL to 0.01 μg/mL (295.86-0.58 nM)) was added into IL-4 R alpha: IL-4-Biotin binding reactions. The assay was performed according to the above-described protocol. Background was subtracted from data points prior to log transformation and curve fitting (QC tested).
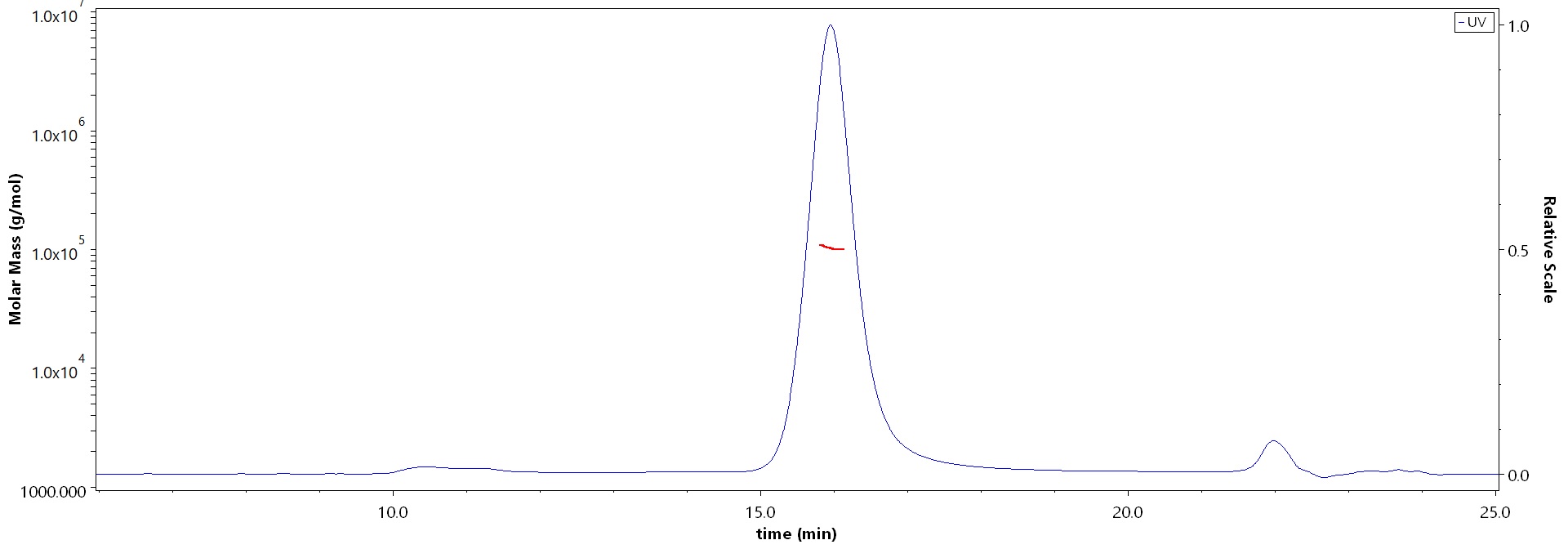
The purity of Human IL-4 Protein, Fc Tag (Cat. No. IL4-H5253) is more than 90% and the molecular weight of this protein is around 90-110 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Berdazimer sodium-SB-206 (Novan) | NVN-1000-SB-206-Novan; MAP3-NONOate - SB-206; NVN1000-SB206; SB-206; SB-019; NI-MC101 | Approved | Zelsuvmi, ZELSUVMI, KINSOLUSTM | United States | Molluscum Contagiosum | Lnhc Inc | 2024-01-05 | Acne Vulgaris; Tinea Pedis; Psoriasis; Molluscum Contagiosum; Condylomata Acuminata; Dermatitis, Atopic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Berdazimer sodium-SB-204 (Novan) | MAP3-NONOate-SB-204; NVN1000-SB204; SB-204 | Phase 3 Clinical | University Of North Carolina At Chapel Hill | Acne Vulgaris | Details |
| Eblasakimab | MK-6105; ASLAN-004; CSL-334 | Phase 2 Clinical | Csl Ltd, Merck Sharp & Dohme Corp | Dermatitis, Atopic; Hypersensitivity | Details |
| Dectrekumab/VAK-694 | QBX-258 | Phase 2 Clinical | Novartis Pharma Ag | Lymphedema; Asthma | Details |
| OCH-NCNP1 | OCH-NCNP1 | Phase 2 Clinical | Keio University | Multiple Sclerosis; Crohn Disease | Details |
| PF-07264660 | PF-07264660 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| PF-07275315 | PF-07275315 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| AUP1602-C | AUP-16; AUP1602-C | Phase 2 Clinical | Aurealis | Diabetic Foot; Ulcer | Details |
| KBL-693 | KBL-693 | Phase 1 Clinical | Kobiolabs | Asthma | Details |
This web search service is supported by Google Inc.
