 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
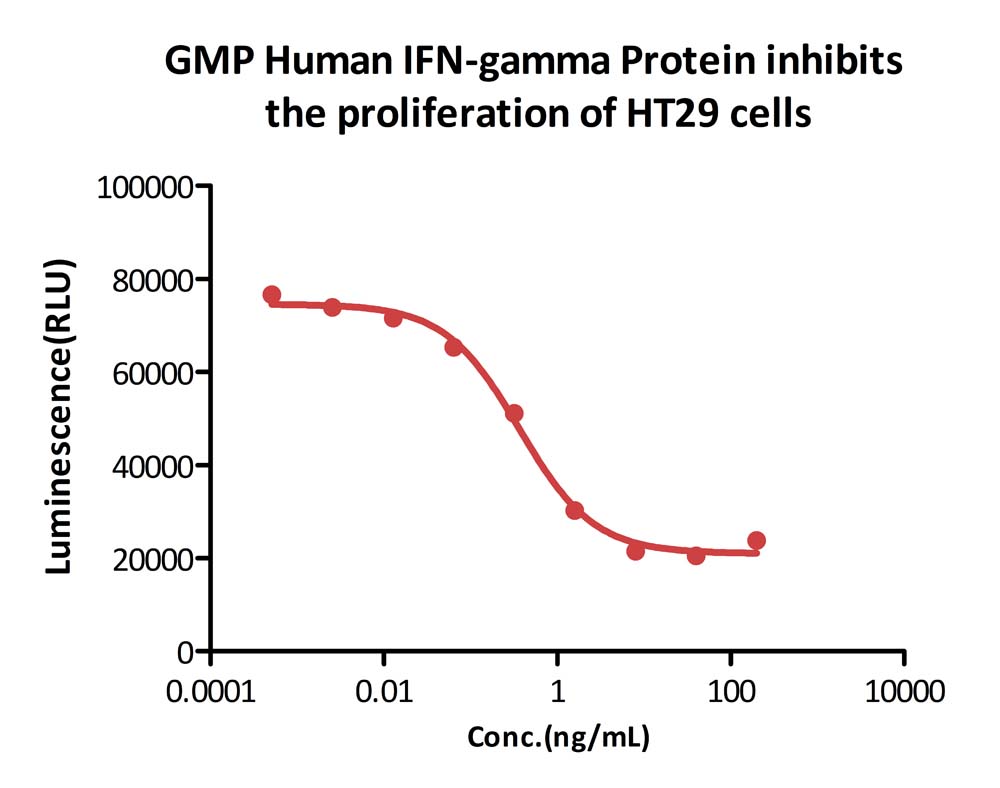
GMP Human IFN-gamma Protein (Cat. No. GMP-IFGH24) inhibits the proliferation of HT-29 cells. The specific activity of GMP Human IFN-gamma Protein is > 2.00×10^7 IU/mg, which is calibrated against human interferon gamma Standard (NIBSC code: 87/586) (QC tested).
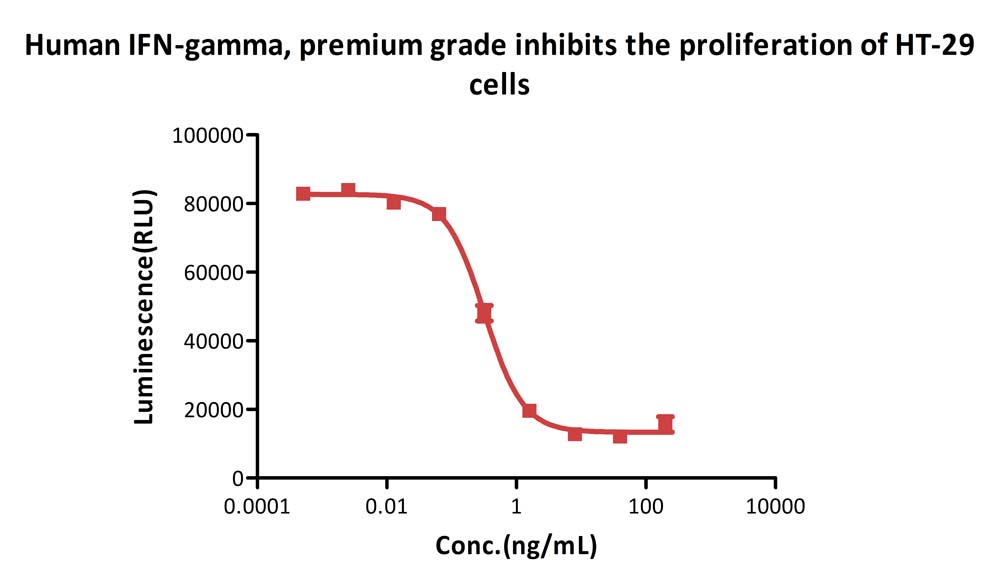
Human IFN-gamma, premium grade (Cat. No. IFG-H4211) inhibits the proliferation of HT-29 cells. The specific activity of Human IFN-gamma, premium grade is > 2.00×10^7 IU/mg, which is calibrated against human interferon gamma Standard (NIBSC code: 87/586) (QC tested).
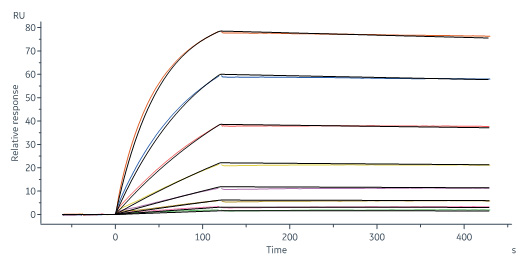
Biotinylated Monoclonal Anti-IFNγ Antibody, Mouse IgG1 (13E6H6) (Cat. No. IFN-BS138) captured on CM5 chip via anti-mouse antibodies surface can bind Human IFN-gamma, premium grade (Cat. No. IFG-H4211) with an affinity constant of 1.28 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
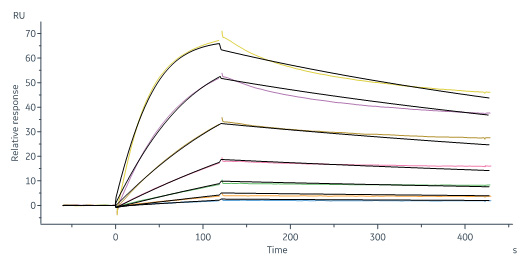
Monoclonal Anti-IFNγ antibody, Mouse IgG2a (8C5F8) (Cat. No. IFN-S120) captured on CM5 chip via anti-mouse antibodies surface can bind Human IFN-gamma, premium grade (Cat. No. IFG-H4211) with an affinity constant of 0.933 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Cadi-05 | Cadi-05 | Approved | National Institute Of Allergy And Infectious Diseases (Niaid) | Immuvac, Leprovac, Mycidac-C | India | Carcinoma, Non-Small-Cell Lung; Leprosy | null | 1998-01-01 | Leprosy; Carcinoma, Non-Small-Cell Lung | Details |
| Emapalumab | NI-0501 | Approved | Novimmune Sa | Gamifant | United States | Lymphohistiocytosis, Hemophagocytic | Novimmune Sa | 2018-11-20 | Macrophage Activation Syndrome; Still's Disease, Adult-Onset; Rare Diseases; Immune System Diseases; Arthritis, Juvenile; Lupus Erythematosus, Systemic; Lymphohistiocytosis, Hemophagocytic | Details |
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Tadekinig alfa (AB2 Bio) | Phase 3 Clinical | Ab2 Bio Ltd | Macrophage Activation Syndrome; Cytokine Release Syndrome; Lymphohistiocytosis, Hemophagocytic | Details | |
| PDS-0101 | PDS-0101B; PDS-0101C; PDS-0101; PDS-0101A; PDS-101 | Phase 2 Clinical | Pds Biotechnology Corporation, Merck Serono | Head and Neck Neoplasms; Anus Neoplasms; Papillomavirus Infections; Vulvar Neoplasms; Oropharyngeal Neoplasms; Carcinoma, Squamous Cell; Uterine Cervical Neoplasms | Details |
| YH-32367 | ABL105; YH-32367 | Phase 2 Clinical | Abl Bio Inc | Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| OCH-NCNP1 | OCH-NCNP1 | Phase 2 Clinical | Keio University | Multiple Sclerosis; Crohn Disease | Details |
| KIO-101(Kiora) | PP-001; KIO-101 | Phase 2 Clinical | 4sc Ag | Dry Eye Syndromes; Eye Diseases; Keratoconjunctivitis; Uveitis; Xerophthalmia; Conjunctival Diseases | Details |
| ASN-002 (Ascend Biopharmaceuticals) | TG-1042; Ad-IFNγ; SP-002 (Ascend Biopharmaceuticals) | Phase 2 Clinical | Transgene Sa | Solid tumours; Lymphoma, B-Cell; Carcinoma, Basal Cell; Skin Neoplasms; Basal Cell Nevus Syndrome; Melanoma | Details |
| IFN-gamma-secreting HAdV antigen specific T cells (Case Comprehensive Cancer Center) | Phase 1 Clinical | Case Comprehensive Cancer Center | Hematopoietic stem cell transplantation (HSCT) | Details | |
| Recombinant human interferon gamma adenovirus injection (Guangzhou Dabo Biological Products) | Phase 1 Clinical | Guangzhou Dabo Biological Products Co Ltd | Liver Neoplasms; Nasopharyngeal Neoplasms; Prostatic Neoplasms, Castration-Resistant | Details | |
| EI-001 | EI-001 | Phase 1 Clinical | Elixiron Immunotherapeutics Inc | Vitiligo | Details |
This web search service is supported by Google Inc.
