 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| GMP-ACAH37 | Human | GMP Human Activin A / INHBA Protein | 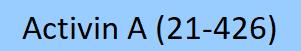 |
||
| ACA-H5314 | Human | Human Activin A / INHBA Protein, premium grade |  |
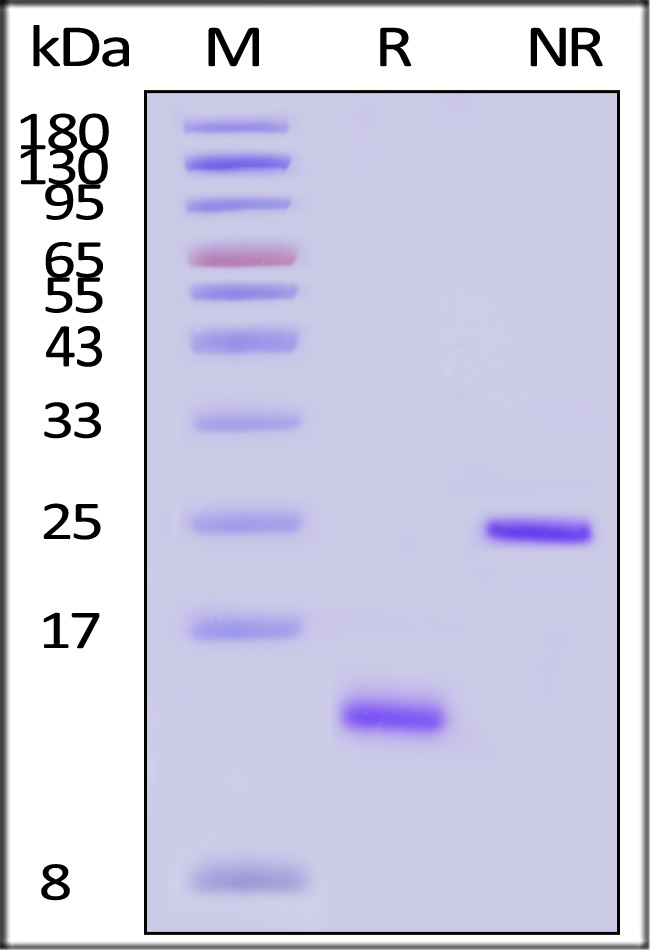
|
|
| ACA-H421b | Human | Human Activin A / INHBA Protein, Tag Free |  |

|
|
| ACA-H424x | Human | Human Latent Activin A / INHBA Protein, His Tag |  |
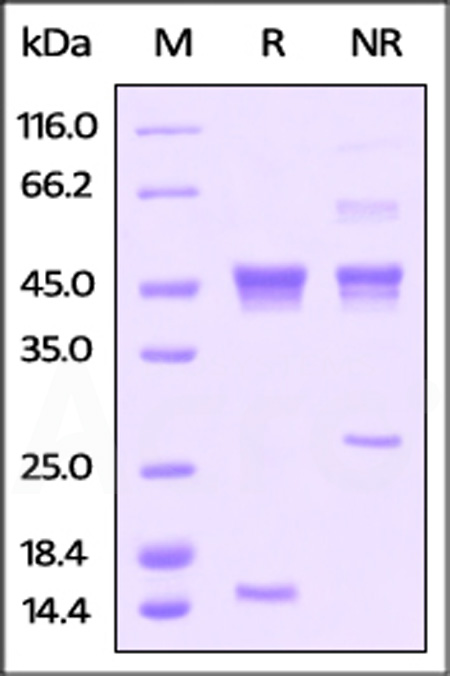
|
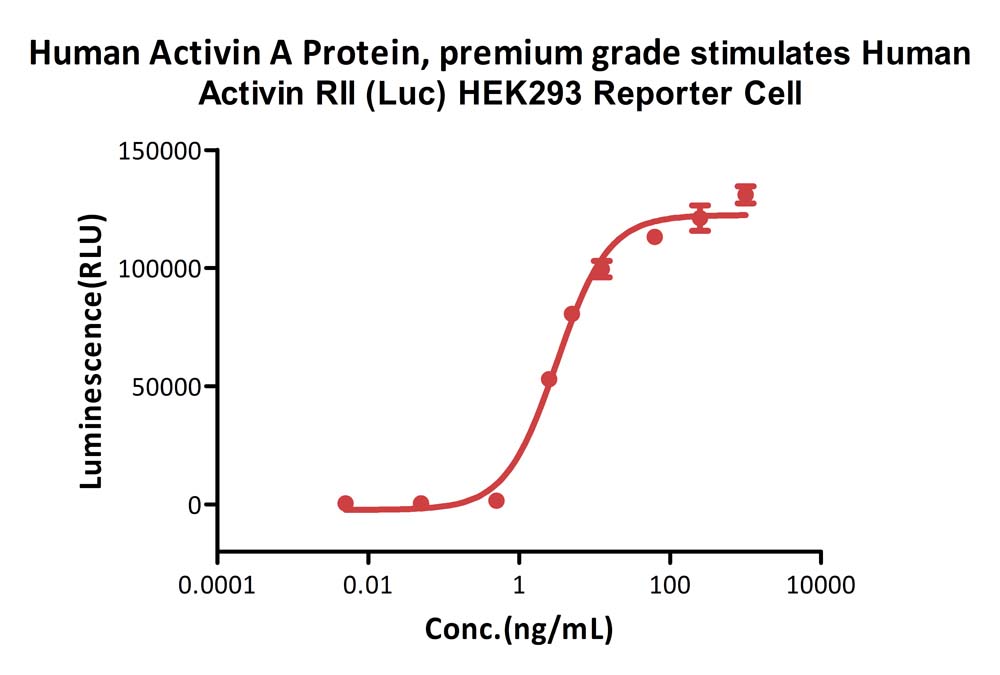
Human Activin A Protein, premium grade (Cat. No. ACA-H5314) stimulates Human Activin RIl (Luc) HEK293 Reporter Cell. The specific activity of Human Activin A Protein, premium grade is>5.00 x 10^2 IU/mg, which is calibrated against WHO Reference Reagent Activin A (Human, Recombinant) NIBSC code: 91/626 (QC tested).
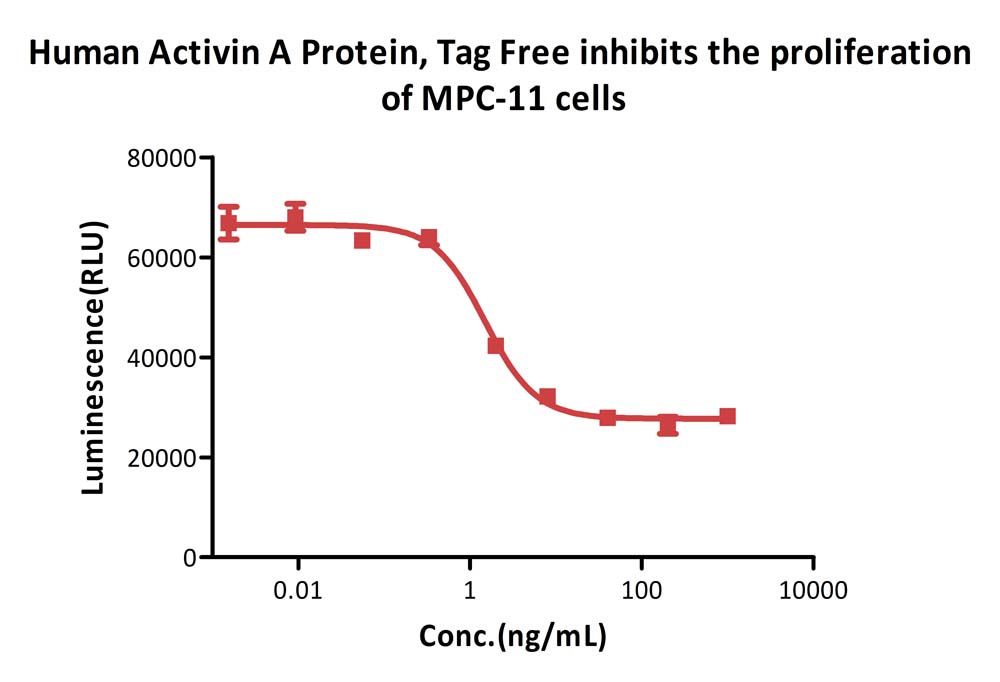
Human Activin A Protein, Tag Free (Cat. No. ACA-H421b) inhibits the proliferation of MPC-11 cells. The specific activity of Human Activin A Protein, Tag Free is > 5.00×10^2 IU/mg, which is calibrated against WHO Reference Reagent Activin A (Human, Recombinant) NIBSC code: 91/626 (Routinely tested).
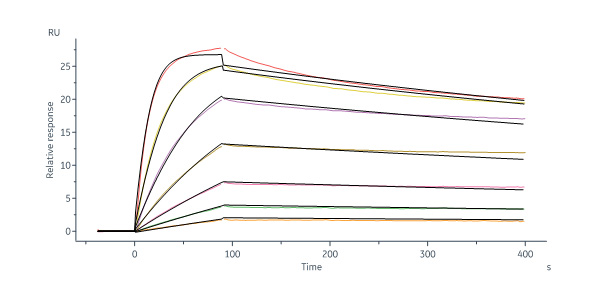
Human Activin RIIB, His Tag (Cat. No. ACB-H5226) immobilized on CM5 Chip can bind Human Activin A Protein, Tag Free (Cat. No. ACA-H421b) with an affinity constant of 0.216 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Momelotinib Dihydrochloride | CYT-387; GS-0387; GSK-3070785 | Approved | Ojjaara, Omjjara | United States | Primary Myelofibrosis; Anemia | Glaxosmithkline Llc, Glaxosmithkline Plc | 2023-09-15 | Polycythemia; Polycythemia Vera; Anemia; Neoplasms; Myeloproliferative Disorders; Pancreatic Intraductal Neoplasms; Primary Myelofibrosis; Carcinoma, Pancreatic Ductal; Splenomegaly; Thrombocythemia, Essential; Carcinoma, Non-Small-Cell Lung | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| BLU-782 | BLU-782; IPN-60130 | Phase 2 Clinical | Blueprint Medicines Corp, Ipsen SA | Myositis Ossificans | Details |
| Zilurgisertib | INCB-000928; INCB-00928 | Phase 2 Clinical | Incyte Corp | Anemia; Polycythemia Vera; Myelodysplastic Syndromes; Kidney Diseases; Ossification, Heterotopic; Multiple Myeloma; Myositis Ossificans; Primary Myelofibrosis; Thrombocythemia, Essential | Details |
| KER-047 | KER-047 | Phase 1 Clinical | National Center For Advancing Translational Sciences | Anemia, Iron-Deficiency; Myositis Ossificans | Details |
| Itacnosertib | TP-0184 | Phase 1 Clinical | Tolero Pharmaceuticals Inc, Sumitomo Dainippon | Solid tumours; Anemia | Details |
| BCX-9250 | BCX-9250 | Phase 1 Clinical | Biocryst Pharmaceuticals Inc | Myositis Ossificans | Details |
This web search service is supported by Google Inc.
