 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| IL5 mAb | Monospecific antibody | Immunological disease | Asthma | Phase II | Global (except China) |
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| IL5-H5214 | Human | Human IL-5 Protein, premium grade | 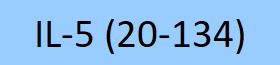 |
|
|
| EP-128 | Human | IL-5[Biotinylated]:IL-5Rα Inhibitor Screening ELISA Kit | |||
| IL5-C52H3 | Cynomolgus | Cynomolgus IL-5 Protein, His Tag |  |
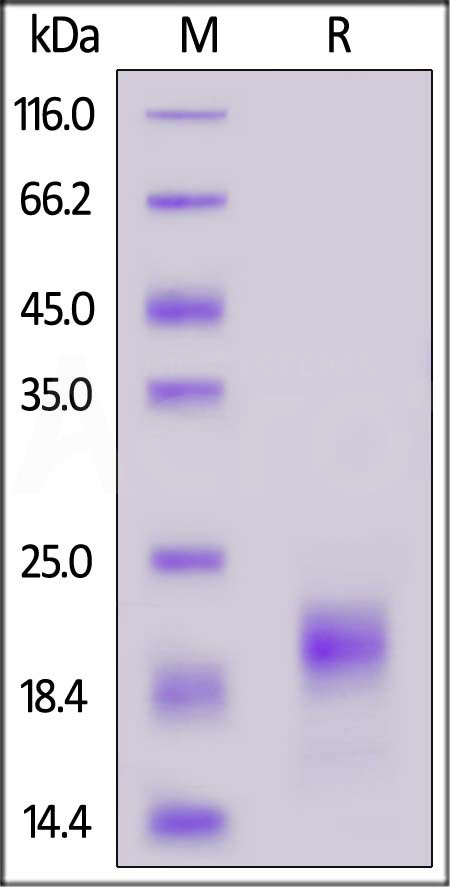
|
|
| IL5-M52H3 | Mouse | Mouse IL-5 Protein, His Tag (MALS verified) |  |
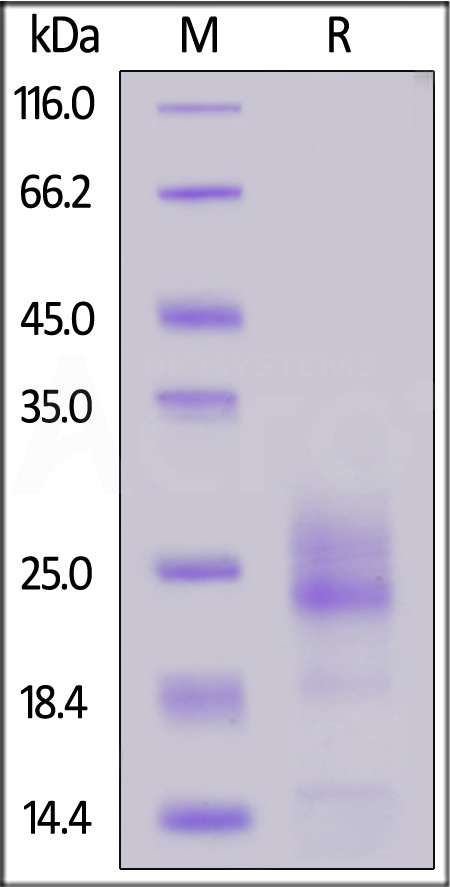
|
|
| IL5-R52H4 | Rabbit | Rabbit IL-5 Protein, His Tag |  |
|
|
| IL5-H82Q5 | Human | Biotinylated Human IL-5 Protein, His,Avitag™ |  |
|
|
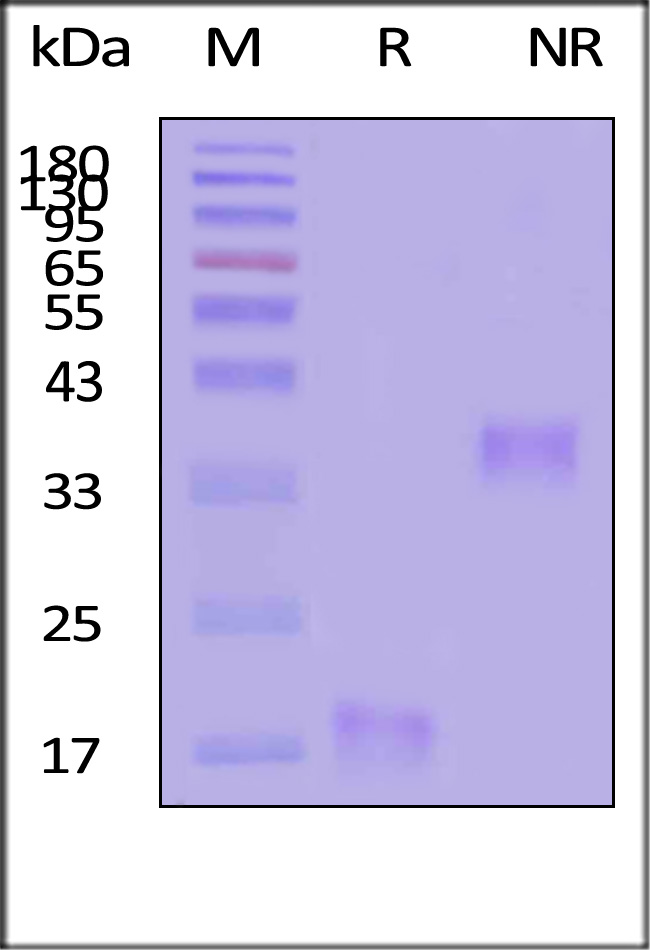
| IL5-H52H3 | Human | Human IL-5 Protein, His Tag |  |
|
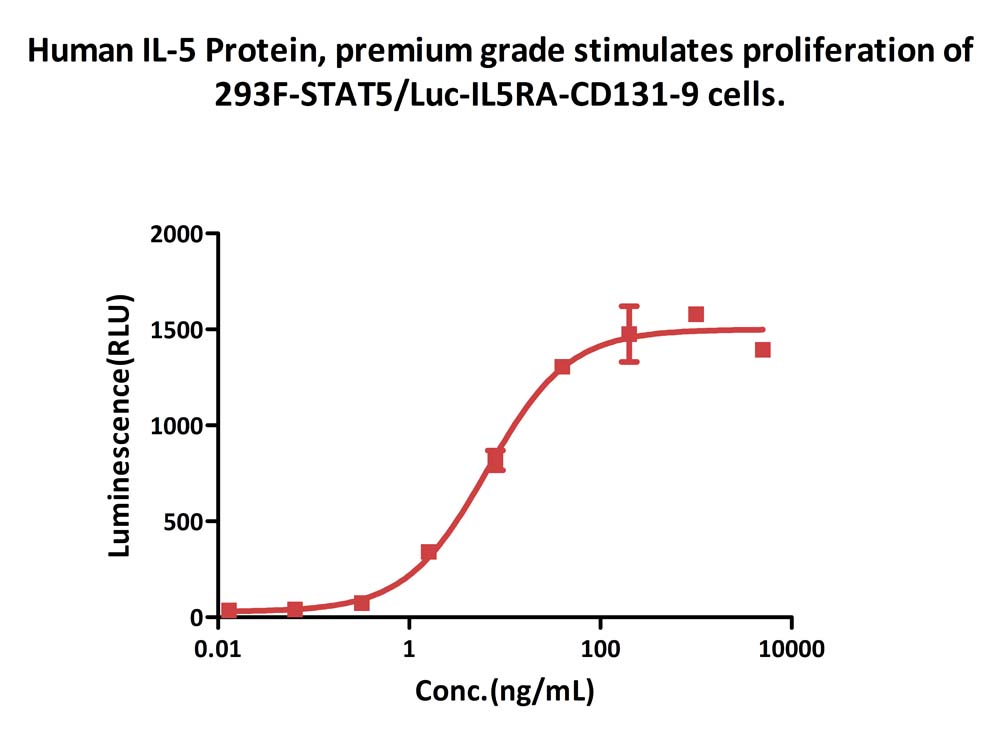
Human IL-5 Protein, premium grade (Cat. No. IL5-H5214) stimulates proliferation of 293F-STAT5/Luc-IL5RA-CD131-9 cells. The specific activity of Human IL-5 Protein, premium grade is > 3.00ⅹ10^6 IU/mg, which is calibrated against human IL-5 WHO International Standard (NIBSC code: 90/586) (QC tested).
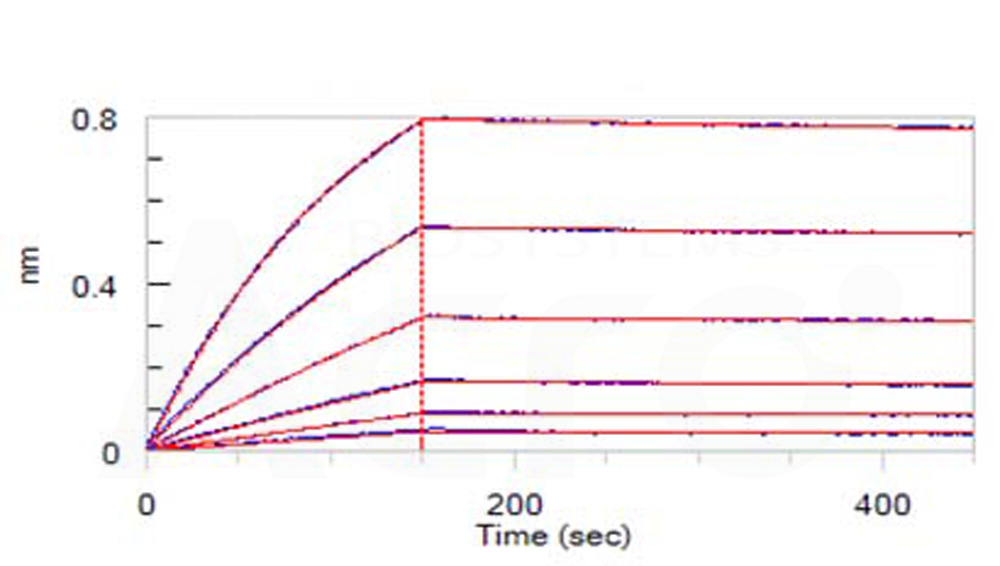
Loaded Human IL-5 Protein, His Tag (Cat. No. IL5-H52H3) on NTA Biosensor, can bind Human IL-5 R alpha, Fc Tag with an affinity constant of 4.63 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
![IL-5[Biotinylated]:IL-5Rα Inhibitor Screening ELISA KitIL-5[Biotinylated]:IL-5Rα Inhibitor Screening ELISA Kit (Cat. No. EP-128) ELISA bioactivity](/static/main/products/images/elisa/EP-128-E1.jpg)
INHIBITION OF IL-5 [BIOTINYLATED]: IL-5 R ALPHA BINDING BY Human IL-5
Serial dilutions of Human IL-5(Catalog # EP128-C03) (1:1 serial dilution, from 10 μg/mL to 0.020 μg/mL (666.67-0.65 nM)) was added into IL-5 R alpha: IL-5-Biotin binding reactions. The assay was performed according to the above-described protocol. Background was subtracted from data points prior to log transformation and curve fitting (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Reslizumab | TRFK-5; CDP-835; SCH-5570; CEP-38072; CTx-55700; SCH-55700 | Approved | Teva Pharmaceutical Industries Ltd | Cinqair, Cinquil, Cinqaero | United States | Asthma | Teva Respiratory Llc | 2016-03-23 | Hypereosinophilic Syndrome; Loiasis; Pulmonary Eosinophilia; Eosinophilic Esophagitis; Asthma; Bronchitis | Details |
| Mepolizumab | SB-240563; 240563 | Approved | Glaxosmithkline Plc | Bosatria, Nucala, 美泊利珠单抗 | United States | Asthma | Glaxosmithkline Llc | 2015-11-04 | Granulomatosis with Polyangiitis; Hypereosinophilic Syndrome; Eosinophilic Granulomatosis With Polyangiitis; Nasal Polyps; Eosinophilia; Chronic Urticaria; Nose Diseases; Virus Diseases; Eosinophilic Esophagitis; Sinusitis; Asthma; Bronchitis; Pulmonary Disease, Chronic Obstructive; Fasciitis; Dermatitis, Atopic; Angioedema | Details |
| Reslizumab | TRFK-5; CDP-835; SCH-5570; CEP-38072; CTx-55700; SCH-55700 | Approved | Teva Pharmaceutical Industries Ltd | Cinqair, Cinquil, Cinqaero | United States | Asthma | Teva Respiratory Llc | 2016-03-23 | Hypereosinophilic Syndrome; Loiasis; Pulmonary Eosinophilia; Eosinophilic Esophagitis; Asthma; Bronchitis | Details |
| Mepolizumab | SB-240563; 240563 | Approved | Glaxosmithkline Plc | Bosatria, Nucala, 美泊利珠单抗 | United States | Asthma | Glaxosmithkline Llc | 2015-11-04 | Granulomatosis with Polyangiitis; Hypereosinophilic Syndrome; Eosinophilic Granulomatosis With Polyangiitis; Nasal Polyps; Eosinophilia; Chronic Urticaria; Nose Diseases; Virus Diseases; Eosinophilic Esophagitis; Sinusitis; Asthma; Bronchitis; Pulmonary Disease, Chronic Obstructive; Fasciitis; Dermatitis, Atopic; Angioedema | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Recombinant anti-IL-5 humanized monoclonal antibody (Shanghai CP Guojian) | 610; SSGJ-610 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Autoimmune Diseases; Asthma | Details |
| SHR-1703 | SHR-1703 | Phase 3 Clinical | Suzhou Suncadia Biopharmaceuticals Co Ltd, Jiangsu Hengrui Medicine Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Eosinophilic Granulomatosis With Polyangiitis; Asthma | Details |
| Depemokimab | GSK-294; GSK3511294; GSK294; GSK-3511294 | Phase 3 Clinical | Glaxosmithkline Plc | Hypereosinophilic Syndrome; Eosinophilic Granulomatosis With Polyangiitis; Nasal Polyps; Vasculitis; Asthma; Eosinophilic Granuloma; Sinusitis | Details |
| long-acting anti-IL-5 mAb (GSK) | Phase 1 Clinical | Glaxosmithkline Plc | Asthma | Details | |
| KBL-693 | KBL-693 | Phase 1 Clinical | Kobiolabs | Asthma | Details |
| Mepolizumab biosimilar(Bio Thera Solutions) | BAT2606; BAT-2606 | Phase 1 Clinical | Bio-Thera Solutions Ltd | Nasal Polyps; Autoimmune Diseases; Sinusitis; Asthma | Details |
| RC-1416 | Phase 1 Clinical | Nanjing Rongjiekang Biotechnology Co Ltd | Asthma | Details | |
| Mepolizumab biosimilar (CTTQ Pharma) | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Granulomatosis with Polyangiitis; Hypereosinophilic Syndrome; Vasculitis; Nasal Polyps; Asthma; Eosinophilic Granuloma; Sinusitis; Lymphohistiocytosis, Hemophagocytic | Details | |
| Recombinant anti-IL-5 humanized monoclonal antibody (Shanghai CP Guojian) | 610; SSGJ-610 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Autoimmune Diseases; Asthma | Details |
| SHR-1703 | SHR-1703 | Phase 3 Clinical | Suzhou Suncadia Biopharmaceuticals Co Ltd, Jiangsu Hengrui Medicine Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Eosinophilic Granulomatosis With Polyangiitis; Asthma | Details |
| Depemokimab | GSK-294; GSK3511294; GSK294; GSK-3511294 | Phase 3 Clinical | Glaxosmithkline Plc | Hypereosinophilic Syndrome; Eosinophilic Granulomatosis With Polyangiitis; Nasal Polyps; Vasculitis; Asthma; Eosinophilic Granuloma; Sinusitis | Details |
| long-acting anti-IL-5 mAb (GSK) | Phase 1 Clinical | Glaxosmithkline Plc | Asthma | Details | |
| KBL-693 | KBL-693 | Phase 1 Clinical | Kobiolabs | Asthma | Details |
| Mepolizumab biosimilar(Bio Thera Solutions) | BAT2606; BAT-2606 | Phase 1 Clinical | Bio-Thera Solutions Ltd | Nasal Polyps; Autoimmune Diseases; Sinusitis; Asthma | Details |
| RC-1416 | Phase 1 Clinical | Nanjing Rongjiekang Biotechnology Co Ltd | Asthma | Details | |
| Mepolizumab biosimilar (CTTQ Pharma) | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Granulomatosis with Polyangiitis; Hypereosinophilic Syndrome; Vasculitis; Nasal Polyps; Asthma; Eosinophilic Granuloma; Sinusitis; Lymphohistiocytosis, Hemophagocytic | Details |
This web search service is supported by Google Inc.
