
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Choose the development strategy of anti-idiotypic antibody to seize the advantage of PK/ADA assay In the past two decades, the rapid development of biological therapeutics, such as CAR-T cell therapy, antibodies, antibody-drug conjugates, etc., has played a vital role in treating many major diseases. However, as an exogenous entity, these drugs may produce anti-drug antibodies after being injected into the body, which may reduce the drug's efficacy and, in severe cases, may be life-threatening. Therefore, Immunogenicity (ADA) studies and pharmacokinetic (PK) analysis are critical in developing biopharmaceuticals.
Anti-idiotypic antibodies are the key tool reagents for PK/ADA assay. Obtaining an accurate and effective bioassay method to ensure the completion of a project on time is important in defining the development strategy of an anti-idiotic antibody. This article summarizes the development strategies of anti-idiotypic antibodies for different types of biological drugs to help you successfully develop your bioanalytical methods.
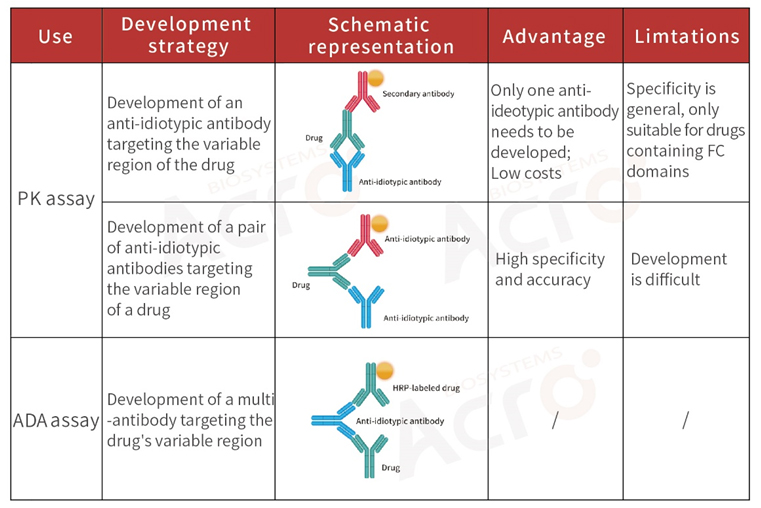
>> Development strategy of anti-idiotypic antibodies for monoclonal antibody drugs
Decades of development, monoclonal antibodies (mAb) have gradually transformed from scientific research tools (reagents) to biological drugs that provide a new therapeutic modality for a variety of diseases. Developing anti-idiotypic antibodies is important in studying a mAbs effectiveness and safety.
>> Development strategy of anti-idiotypic antibody for bispecific antibodies
Bispecific antibody: A modified antibody containing two specific antigen-binding sites. The pharmacokinetic analysis of the drug is more complex because of its two distinct variable regions.
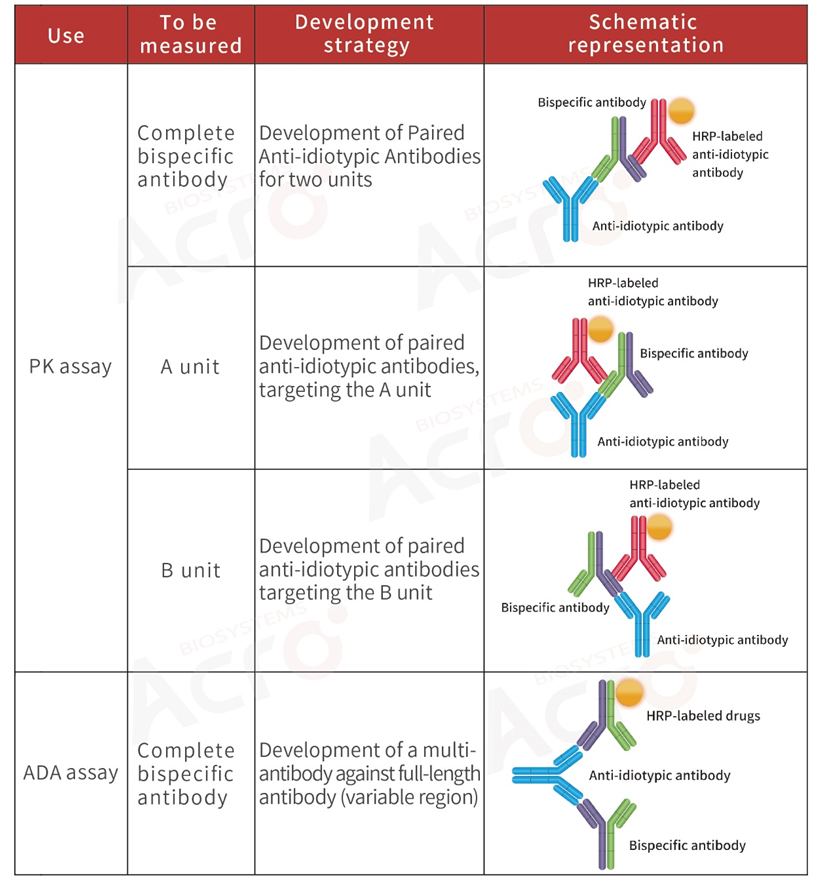
To better evaluate the safety and efficacy of antibody drugs, it is generally recommended that researchers choose to develop anti-idiotypic antibodies for the complete bispecific antibody when performing PK analysis.
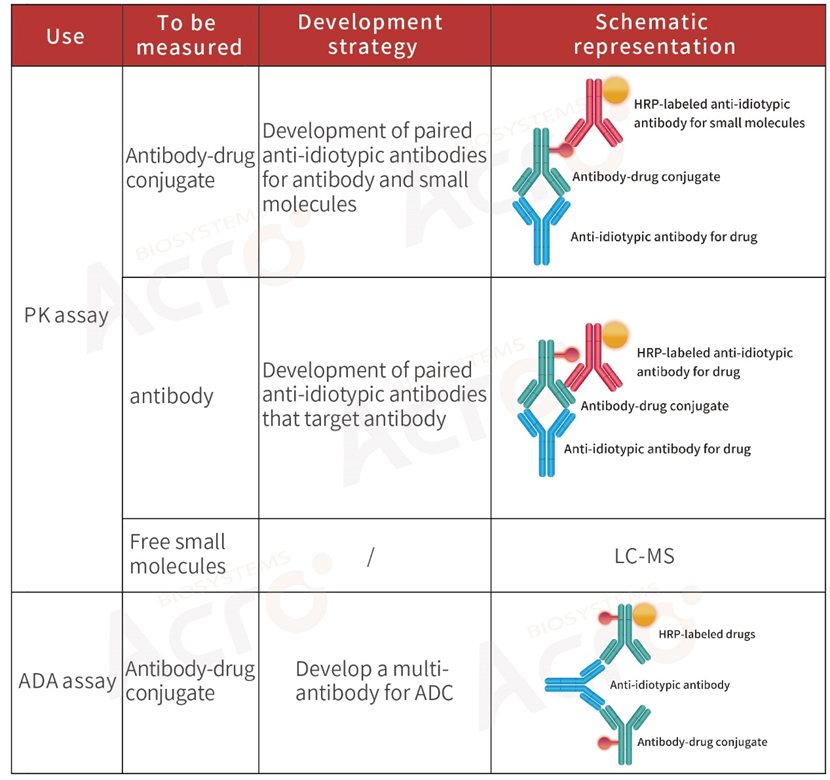
>> Development strategy of anti-idiotypic antibody for ADC drugs
Antibody-drug conjugates (ADCs) are innovative biopharmaceutical products in which a monoclonal antibody is linked to a small molecule drug with a stable linker. Due to the structural specificity and complexity of ADCs, the evaluation of PK for ADCs requires a unique and more complex bioassay protocol compared to conventional small molecular drugs and monoclonal antibodies.
PK analysis of ADCs typically detects three key components: total antibodies, total ADC drugs, and free small molecular cytotoxic drugs (with/without linker). Researchers typically use ligand-binding assay for ADCs and total antibodies to assess the effectiveness and targeted toxicity of ADCs. For the safety and stability assessment of ADCs, that is, the toxicity of such drugs to non-tumor cells in vivo, it is necessary to quantify the free small molecular toxicity.
>> Development strategy of anti-idiotypic antibody for CAR-T drugs
Chimeric antigen receptor T (CAR-T) cell immunotherapy. CAR-T cells are the antigen-binding parts of antibodies that can recognize a specific tumor antigen with the intracellular part of the CD3-ζ chain or FcεRIγ in vitro coupled to a chimeric protein. CAR was expressed in T cells of patients by gene transduction. For CAR-T, real-time fluorescence quantitative polymerase chain reaction (qPCR) and flow cytometry (FCM) were used for PK analysis to determine the foreign gene copy and the number of CAR + cells, respectively. Cell-based assays are used to screen anti-idiotypic antibodies against genetically engineered scFv antibodies for PK analysis of CAR-T.

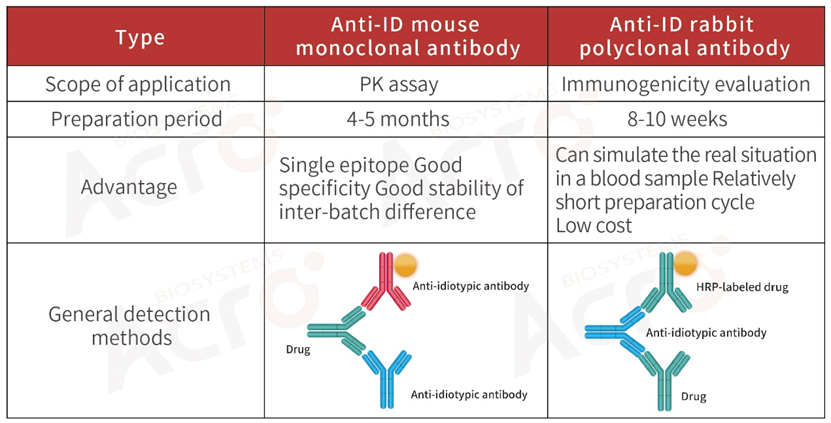
>> Development strategy of anti-idiotypic antibody for biosimilar
For the PK / ADA assays of biosimilar drugs, anti-idiotypic antibodies can be developed according to the variable region of the drug, or the original ADA product can be purchased.
ACROBiosystems has developed a series of high-affinity, high-specific anti-idiotypic antibodies and PK blood concentration quantitative detection kits for immunogenicity analysis and pharmacokinetic studies to support these studies. We will develop the corresponding protocol according to the different application scenarios for each anti-idiotypic antibody, hoping to speed up the drug development process to the greatest extent. The products developed cover adalimumab, rituximab, cetuximab, trastuzumab, bevacizumab, and many other popular antibody drugs.
ACROBiosystems, as a global brand of protein technologies, products, and services focused on biologics, is committed to providing targeted antigens, other key reagents and related services required in the development process of targeted therapeutic drugs. To meet the diverse needs of customers, we can also provide one-stop service from antigen preparation to monoclonal anti-idiotypic antibodies, polyclonal anti-idiotypic antibody, pharmacokinetics, and immunogenicity test kit development.
Contact us: You can fill in the inquiry form onlineAnti-Idiotypic Antibody development service inquiry and we will respond to your needs within 24 hours.
This web search service is supported by Google Inc.








