Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > ACROBiosystems announces the release of their latest BA.2.75 antigen products 
Last week, ACROBiosystems announced their latest antigen products targeting BA.2.75, an emerging Omicron mutation.
BA.2.75 has been spreading exponentially throughout India since May. Shortly after the discovery of this new mutation, new related cases have been detected in many other countries, including the UK, US, Australia, Germany, etc. Unofficially nicknamed “Centaurus”, BA.2.75 has been classified as a Variant of Interest (VOIs) instead of a Variant of Concern (VOCs) by the World Health Organization (WHO). Although the threat of this emerging Omicron mutation is still unclear, its presence keeps medical experts across the globe on alert.
Dr. Maria Van Kerkhove, the WHO’s COVID-19 technical lead, indicated that there are an insufficient number of samples for the proper analysis of BA.2.75. Currently, only 200 sequences have been collected from 14 countries: a major limiting factor towards the understanding of this mutation.
With a large majority of the population immunized against Covid-19, a high-level of immune evasion has become the norm for newer variants. As such, increased infection rates by novel COVID variants can be expected, despite the significant vaccinated population. This has been similarly confirmed in a recent study by Yamasoba, et al. Out of 10 therapeutic monoclonal antibodies tested against BA.2.75 and other novel variants, most of the approved therapies failed to neutralize the Covid variants, especially BA.2.75.[2]
Unlike the other Omicron subvariants, Centaurus (BA.2.75) has a few extra mutations beyond the same high-level variant structure as Omicron BA.2. Sequence analysis of both variants shows that BA.2.75 contains 17 more nucleotide mutations than BA.2. Several key mutations, G446S and Q493R on the Spike protein, have significant antigen-converting effects. Based on previous studies, the G446S mutation is one of the most effective immune escape sites, while the Q493R mutation may increase the affinity of the virus to the ACE2 receptor.
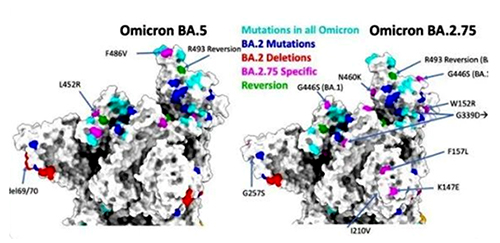
The presence of these novel, more infectious Covid variants has brought into question the long-term vaccine strategy for Covid. More and more experts have begun to accept the concept of utilizing a ‘booster shot’ rather than developing a new vaccine to combat the new variants. Moderna recently announced that two potential boosters are expected to be released this fall targeting the Omicron subvariants.
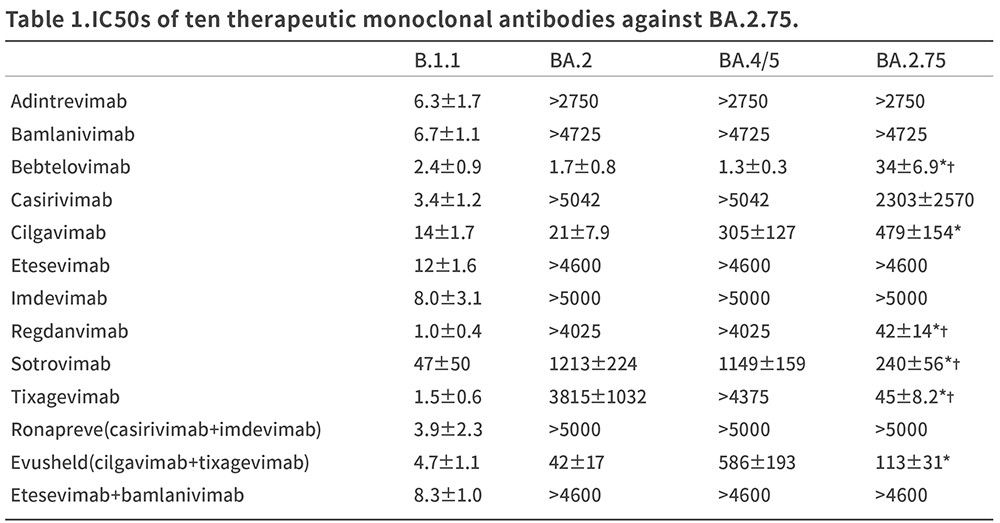
FIGURE 2: Neutralization neutralization assays were performed performed using pseudoviruses harboring the SARS-CoV-2 Spike proteins of BA.2, BA.4/5, and BA.2.75. YAMASOBA .[2]
In the current COVID-19 landscape, the highly contagious BA.5 Omicron subvariant remains as the dominant coronavirus strain in many countries. According to the data from the Global Initiative on Sharing All Influenza Data (GISAID), BA.5 accounted for 52% of the sequence library. Furthermore, the study shows that the R0 value, a nominal value denoting infectiousness, is 18.6. This is around 6-fold of the wild-type, making it the most infectious subvariant of the SARS-CoV-2 virus.
In order to assist the development of vaccines targeting newer Covid variants and responding to the rapid demands of the market, ACROBiosystems’ latest BA.2.75 antigen-related products have been released. With the development of COVID-19, ACROBiosystems strives to continuously supply the evolving essential antigens for vaccine research, including the Spike timer, S RBD, and Nucleocapsid protein.
- Covers almost all new Omicron subvariants: BA.4/5, BA.2.74/75/76, BA.2.38 etc.;
- Offer Spike trimer, S RBD, S1, S2, NTD and N protein in biotinylated and unconjugated versions are available;
- purity is more than 95% verified by SDS-PAGE and over 90% determined by MALS;
- High bioactivity verified by strict quality control, which is suitable for ELISA/SPR/BLI and other experiments.
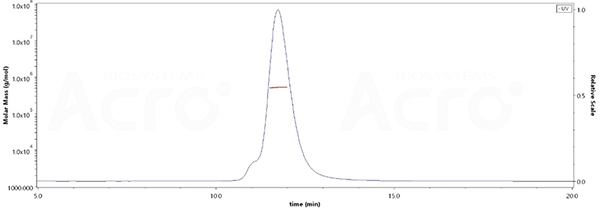
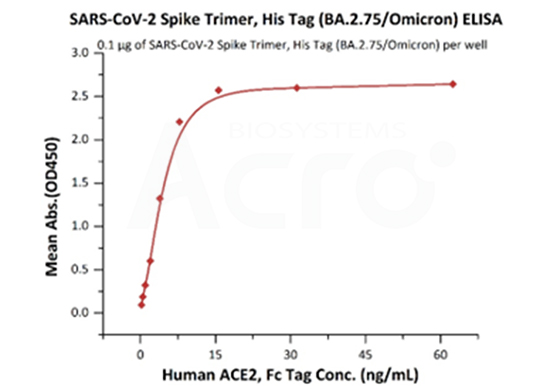
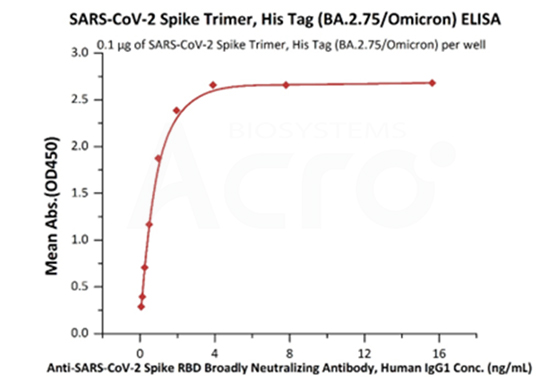
The purity of SARS-CoV-2 Spike Trimer, His Tag (BA.2.75/Omicron) (Cat. No. SPN-C522f) is more than 90% verified by SEC-MALS. The molecular weight of this protein is around 496-548 kDa.
1. Topol, E., 2022. BA.5, Chapter 2. [online] Erictopol.substack.com. Available at: https://erictopol.substack.com/p/ba5-chapter-2.
2. Yamasoba D, Sato K, et al, 2022. Neutralization sensitivity of Omicron BA.2.75 to therapeutic monoclonal antibodies. The Lancet Infectious Diseases.
This web search service is supported by Google Inc.