Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > The Race Against Cancer: Exploring the Need for Innovative Immune Checkpoint Therapy Options 
Many hematological and solid malignancies can escape our natural antitumor immunity by disrupting immune checkpoint (IC) expression. Immune checkpoint (IC) inhibitors, a promising immunotherapy, re-regulates the activity of cytotoxic T lymphocytes and natural killer cells re-establishing and enhancing innate immunity. Therapies targeting ICs such as cytotoxic T-lymphocyte antigen (CTLA-4), programmed cell death (PD-1), and its ligand (PD-L1), have been approved by the FDA to treat various cancers.1
Despite their promise, IC therapies often suffer from non-response in most patients, a condition termed primary resistance. Other patients who respond initially, can develop resistance and experience tumor relapse, defined as acquired resistance.2Altogether, only a small percentage of patients is responsive to IC inhibitor therapy, limiting their clinical application. Thus, focus has shifted to exploring the underlying mechanisms responsible and finding new ICs or combination treatment strategies to improve treatment efficacy.
IC inhibitor resistance mechanisms are classified as either primary or acquired. They encompass cancer cell alterations, including immune recognition, cell signaling, gene expression, DNA damage response and T-cell activation process.3 Currently, several resistance mechanisms have been identified. Within this article, the major mechanisms contributing to primary and acquired resistances are explored.
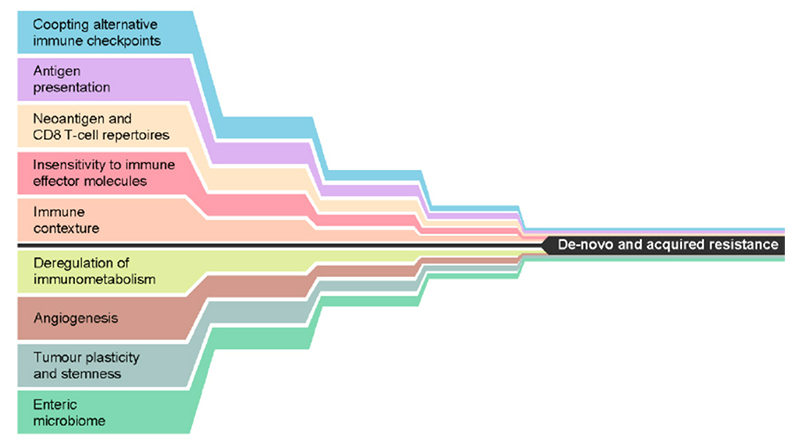
Figure 1. Mechanisms that may either alone, or in combination, lead to de novo or acquired resistance to immune checkpoint inhibition. Reproduced from Fares, et al. 3
Immune contexture, also known as tumor microenvironment, refers to immune factors extrinsic to cancer cells (immune and stromal cells, cytokines, and other biologics that influences therapeutic response). Within this microenvironment, molecular and cellular compositions are altered establishing an immunosuppressive climate. Regulatory T cells (Tregs), myeloid-derived suppressor cells, and tumor-associated macrophages all contribute to this modification through various methods including cytokine secretion, T effector cell suppression, and elevated transforming growth factor beta. This contributes directly to primary resistance of IC inhibitor therapy.
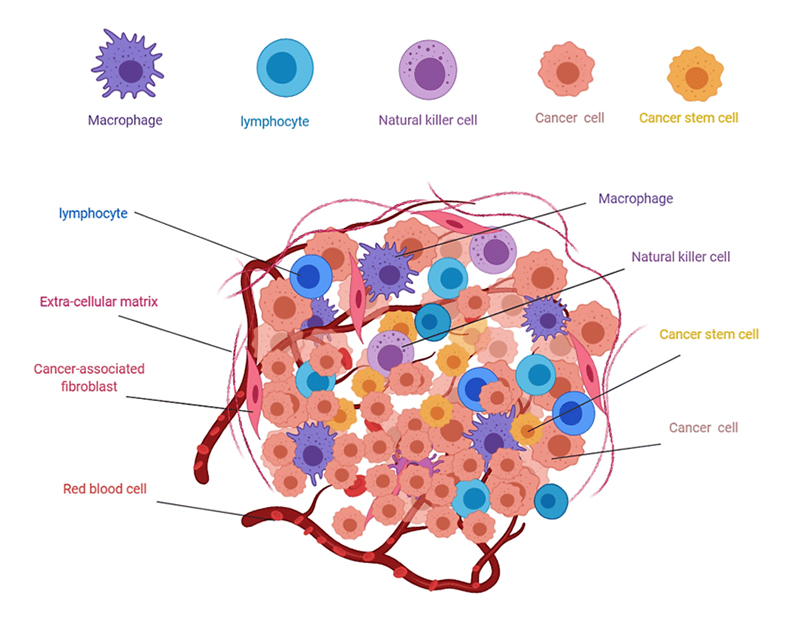
Figure 2.Composition of solid tumors 4
A critical part of our innate immune response is the evolution of response, a process that is often manipulated in tumor malignancies. Unlike the tumor microenvironment which generates an immunosuppressive climate, the modification in immune response inhibits T cell proliferation and diversification.
Co-expression of multiple immune checkpoints results in a severely exhausted T-cell state causing impaired effector function, progressive loss of T cell function, altered transcriptional states, and antigen persistence. In cases of upregulated, co-expressed immune checkpoints, targeting or co-targeting these alternative checkpoint receptors may serve as a potential solution to preventing acquired resistance.
Finally, the last resistance mechanism towards IC therapies is tumor immunogenicity, otherwise known as sensitivity to immune effector molecules. Tumor immunogenicity can be measured by the number of immunogenic neoantigens recognized as foreign or tumor mutational burden (TMB). Heterogenous tumors that interact with the immune system preferentially select low TMB tumors. This phenomenon is a major contributor to acquired resistance, as shown in a study by Anagnostou et al. on relapsed non-small-cell lung carcinomas (NSCLC).4
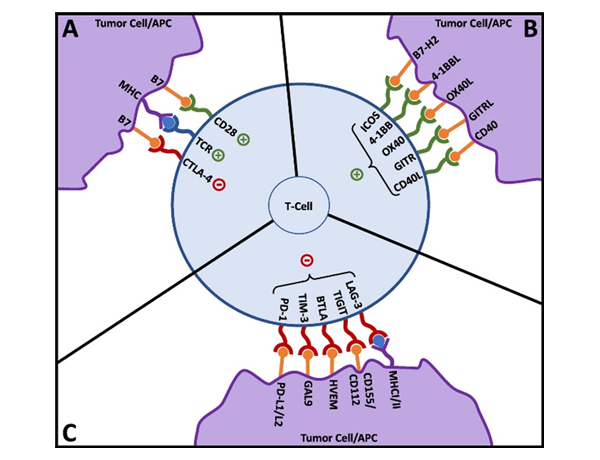
Figure 3. T-cell activation and co-signaling. Reproduced from Fares,et al. 3
Based on the insights highlighted above, the role of ICs in guiding the innate immune system is clear. Over- and under-expression of ICs can be considered as a driving factor in building the immune suppressive TME and altering immune response evolution. However, variation in response to IC inhibitor therapy indicates the complexity of IC inhibitor resistance and the presence of undiscovered mechanisms. As such, developing new IC inhibitor therapies have the potential to overcome resistance mechanisms and expand the limited applications of current immunotherapies.
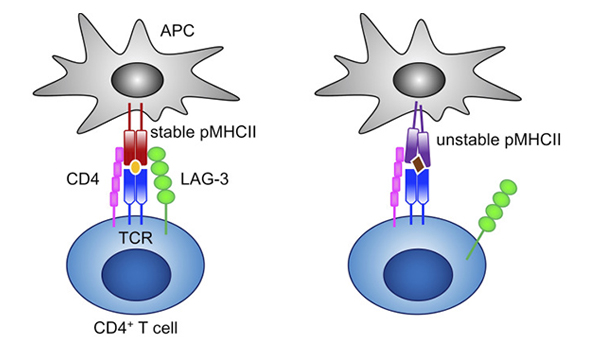
Figure 4.LAG-3 selectively binds to stable pMHCII and inhibits the activation of CD4+ T cells that recognize stable pMHCII. 6
One notable IC inhibitor is LAG-3. LAG-3 is a major immunosuppressive molecule that downregulates T cell cytokine production, CD4/8 expansion, and favors Treg adoption to prevent tissue damage and autoimmunity.5 Blockade of LAG-3 results in a favorable immune activation against tumor cells while further enhancing other IC inhibitors. Clinically, pairing LAG-3 with PD-1 therapy results in an augmented therapeutic effect, whereas LAG-3 monotherapies only give a moderate response.
In March 2022, a LAG-3/PD-L1 combination therapy (Opdualag) was approved by the FDA for unresectable or metastatic melanoma.8 Over 100 clinical trials evaluating LAG-3 inhibitors are ongoing, further highlighting its potential as an IC therapy.
Another potential IC target is TIM-3. TIM3 is expressed on a wide variety of immune cells including CD4/8 T cells, Tregs, myeloid cells, NK cells, and mast cells.9 The cellular diversity of TIM-3 results in immune response modulation through multiple cell pathways. For now, the pathophysiology of TIM-3 in innate immunity remains elusive, however, its role across various cell pathways and the significant preclinical data supports TIM-3 as immune checkpoint, thus revealing itself as a promising treatment method for immunotherapy across various diseases beyond cancer.
Despite the significant potential of immunotherapy, IC inhibitor therapies have found limited use due to the prevalence of primary or acquired resistance and narrow indications for use. In part, this stems from an incomplete understanding of the immune checkpoint and resistance mechanisms, as well as a limited number of actionable immune checkpoints targets for therapeutic use. Despite current gaps in knowledge, clinically and biologically, the initial promising results and durable responses of immunotherapies cannot be ignored. The potential of immunotherapy towards unlocking the secrets of our innate defense defenses is immense.
To help accelerate the research into innovative immunologic therapies, ACROBiosystems is committed to developing and updating a series of IC proteins, inhibitor screening kits, and overexpression cell lines.
1. Archilla-Ortega, A., Domuro, C., Marin-Liberal, J. et al. Blockade of novel immune checkpoints and new therapeuic combinaions to boost anitumor immunity. J Exp Clin Cancer Res 41, 62 (2022).
2. Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. Jan 24;16:223-249. (2021)
3. Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Paients? Am Soc Clin Oncol Educ Book. Jan;39:147-164. (2019)
4. Ghmkin H., Seno M. Blood and Cancer: Cancer Stem Cells as Origin of Hematopoieic Cells in Solid Tumor Microenvironments. Cells 9,1293 (2020)
5. Anagnostou V, Smith KN, Forde PM, et al. Evoluion of neoanigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 7:264-276 (2017)
6.Maruhashi T., Okazuki T., et al. LAG-3: from molecular funcions to clinical applicaions. J. Immunother Cancer. 8(2):e001014 (2020)
7. Marin-Acevedo, J.A., Kimbrough, E.O. & Lou, Y. Next generaion of immune checkpoint inhibitors and beyond. J Hematol Oncol 14, 45 (2021).
8. Food and Drug Administraion. FDA approves Opdualag for unresectable or metastaic melanoma (2022).
9. Wolf, Y., VK Kuchroo, et al. TIM3 comes of age as an inhibitory receptor. Nature Reviews Immunology 20, 173-185 (2020).
This web search service is supported by Google Inc.