
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
When discussing about immune cell therapies, it is almost impossible not to mention cytokine release syndrome (CRS), one of the most well-known adverse effects in immunotherapies. CRS involves the uncontrolled release of pro-inflammatory cytokines including Interleukin (IL)-1, IL-2, IL-6, and TNF-α, etc. When secreted in combination and in excess, these cytokines trigger the rapid onset of fever and other general symptoms that can easily deteriorate into cardiorespiratory organ dysfunction and failures. Within the currently approved cell therapies including chimeric antigen receptor (CAR) T cell therapy, the onset of CRS is nearly universal, where over two-thirds of patients will have symptoms related to CRS and 23% of patients having Grade 3 or higher CRS symptoms. As such, practitioners are constantly seeking to find new ways to manage CRS response, erstwhile researchers have begun to pursue developing safer, cell therapies.
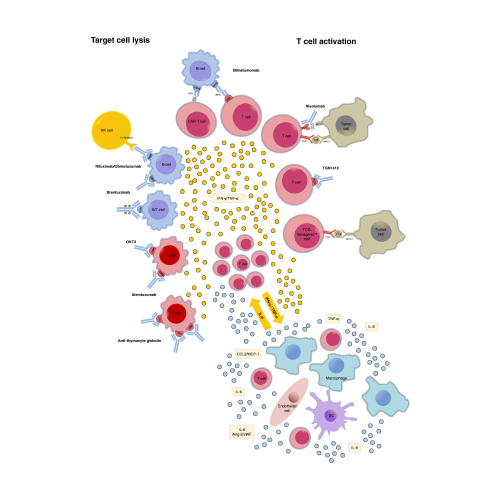
As with any type of pharmaceutical treatment regimen, balancing both therapeutic efficacy and adverse effects is of the utmost priority. When it comes to cancer treatment, especially refractory or relapsed cancers, there are certain calculated risks that enable cell therapies to be approved. However, this comes with several significant limitations: drug exposure time (dosing regimen) and drug concentration (number of infused CAR cells). Due to these limitations, cell therapy treatment strategies are heavily reliant on monitoring CRS onset and severity, both common occurrences that can lead to abandonment of CAR cell infusion.
This brings us to one of the new exploratory regions of cell therapies, CAR-NK. Normally, natural killer (NK) cells are hard to enrich, representing only 10% of lymphocytes, further reducing the efficacy of leukapheresis and straining the manufacturing capacity. Fortunately, a new cell line, NK92, derived from non-Hodgkin’s lymphoma, has been developed, acting as a NK-like cell with pre-activated capabilities. With he advent of NK92 cell line, CAR-NK has begin to enter clinical phases with the general assumption that this type of CAR immune cell does not induce CRS.
However, clarifications must be made – CAR-NK does NOT eliminate cytokine secretion, but rather has a different cytokine secretion profile that does not include the common inducers for CRS (e.g., IL-6). As a result, the occurrence of CRS is diminished but not eliminated, but with the same level of severity; thus, have a similar limitation to other cell therapies.
The occurrence of CRS in experimental CAR NK92 clinical trials have also been reported. In a phase II clinical trial, one patient was observed to have a series of unexpected symptoms progressing into hypotensive shock, high fever, myalgia, and abnormal blood. Upon further analysis of serum cytokine levels, TNF-α and IFN-γ were elevated with no appreciable difference in IL-6. Despite IL-6 being a major driver in the inflammatory symptoms associated with CRS, the high concentration of cytokines can also trigger a CRS-like series of adverse side effects. In essence, CRS is still not comprehensively understood; in part, due to the complex interconnective cytokine signaling pathways, as well as the specific clinical effects of secreted cytokines.
With the development of new, different immune cell therapies, each with a different cytokine secretion profile, another key area of improvement stems from the current CRS management strategies. This includes CRS diagnostics, grading, and suggested treatment strategies. The current algorithm has recently been updated for its implementation in CAR-T clinical trials, focused on clinical symptoms such as fever, organ dysfunction, as well as monitoring at least two cytokine profiles. However, with the advent of CAR-NK, the chosen cytokine profiles that are most likely to initiate CRS remain unclear. Another key issue is the standardized protocol treatment algorithm for severe CRS. Tocilizumab, an FDA-approved antibody that binds to IL-6 receptor, has shown to be extremely effective in ameliorating CRS symptoms: however, the case study above shows no clear elevation of IL-6; thus minimizing the efficacy of a first-line CRS treatment drug. As such, the development of early detection and symptomatic management of CRS unique to NK cells may be also be a focal point towards preventing significant CRS toxicities in next-generation immune cell therapies.
With the increase in CAR-NK and the research into other CAR-NK cell therapies, understanding the cytokine signaling pathway is a must: not only for learning the biological effects, but also in the clinical space for diagnostics and treatment strategies. Identifying immune-cell specific or a set of cytokines can make it easier to diagnose CRS and subsequently monitor using newer, real-time diagnostic technologies. Of course, this means testing a wide range of cytokines beyond the normal CAR-T CRS panel that includes IL-6. ACROBiosystems offers a wide range of various reagents for cytokine detection to support all applications, starting from research and development to clinical research.
>> Click here to learn more about cytokine antibodies, ELISA assay pair and ELISA kits
This web search service is supported by Google Inc.







