 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Universal CAR-NK Cell Therapy: The Promising Future of Targeted Cancer Immunotherapy As critical effectors of the innate immune system, natural killer (NK) cells possess potent anti-tumor capabilities, making them attractive candidates for cancer immunotherapy. With the advent of chimeric antigen receptor (CAR) technology, initially developed for T cells, the potential for engineering CAR-modified NK cells has gained significant attention. While CAR-NK cell therapy builds upon the foundational principles of CAR-T cell therapy, it also incorporates numerous innovations tailored to the unique biological features of NK cells. Although CAR-NK research emerged slightly later than CAR-T, its universal applicability, off-the-shelf availability, and potential efficacy against solid tumors position it as a critical breakthrough in the field of cellular immunotherapy.
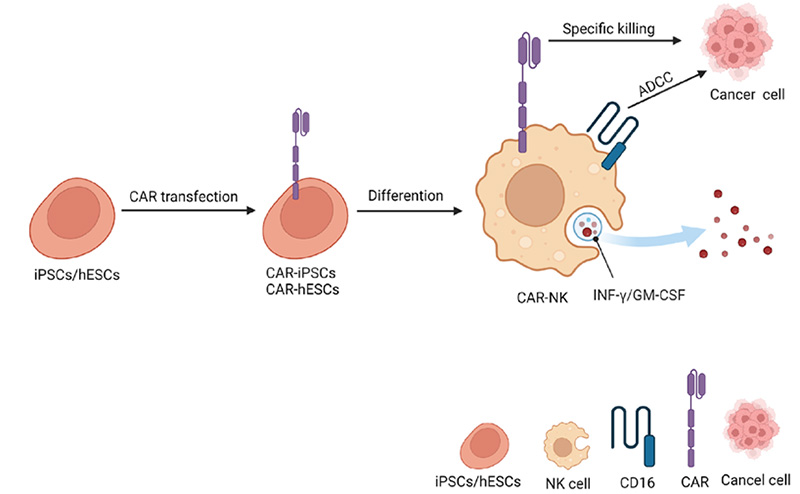
Figure 1. Schemic representation of iPSC-derived CAR-NK allogeneic cell therapy. Differentiation (Hang). 2023; 130:51-57
Excellent Safety Profile
One of the most notable advantages of CAR-NK over autologous CAR-T is their superior safety profile. Although CAR-T cell therapies are effective, they are often linked to severe side effects, including cytokine release syndrome (CRS) and neurotoxicity. In contrast, CAR-NK cells have demonstrated significantly lower rates of CRS and neurotoxicity. This can be partly attributed to NK cells not proliferating to the same extent as T cells upon activation, and their shorter lifespan in circulation, naturally limiting their ability to induce prolonged pro-inflammatory responses. Additionally, the distinct cytokine profiles secreted by these two cell types play a role. While activated NK cells primarily produce IFN-γ and GM-CSF, CAR-T cells elicit cytokines more closely associated with CRS and neurotoxicity, such as IL-2, IL-6, and IL-8.
Broad-Spectrum Anti-Tumor Activity
CAR-NK cells exhibit a broad-spectrum anti-tumor activity that is advantageous over CAR-T therapies. While CAR-T cells are engineered to target a specific antigen on tumor cells, NK cells can recognize and kill tumor cells independently of CAR targeting. This is due to their innate ability to detect stress signals and a lack of human leukocyte antigen (HLA) class I molecules on tumor cells, which are common strategies by which cancer cells evade the immune system. Furthermore, NK cells can mediate antibody-dependent cellular cytotoxicity (ADCC), which CAR-T cells cannot. This means that NK cells can be effective against a wider range of tumors, including those that might develop mechanisms to escape CAR-T cell recognition.
Development of Universal Off-the-Shelf Therapies:
In contrast to most autologous CAR-T cell therapies, which involve complex, time-consuming, and laborious manufacturing processes, CAR-NK cells do not require HLA matching, significantly reducing the risk of allogeneic responses and graft-versus-host disease. Additionally, NK cells can be sourced from various origins, commonly including the NK92 cell line, peripheral blood mononuclear cells (PBMCs), umbilical cord blood (UCB), and induced pluripotent stem cells (iPSCs).
Advantages of iPSC derived NK Cells
Leveraging the innate advantages of NK cells, CAR-NK cell therapy holds promise for the development of allogeneic and scalable products, in contrast to the predominately autologous nature of CAR-T cell therapies. However, the choice of cell source significantly impacts the realization of these characteristics. Primary NK cells, derived from diverse sources, represent a heterogeneous mixture of highly differentiated cells, posing challenges for genetic engineering and ex vivo expansion. Furthermore, identifying and characterizing modified primary cells can be problematic. In contrast, iPSCs are clonal cell lines amenable to various genetic modifications at the stem cell stage, enabling unlimited expansion and controlled differentiation into NK cells, thus forming the foundation for robust and scalable manufacturing processes. Consequently, iPSCs offer significant advantages over other currently available NK cell sources.
iPSC-NK Differentiation Culture
iPSC-NK cell differentiation can be carried out using either 2D or 3D culture systems, each with its own advantages and limitations.
The 2D culture system for NK cell differentiation typically involves co-culturing human PSCs with mouse bone marrow stromal cells or genetically engineered feeder cells expressing Notch ligands (DLL1 or DLL4) to promote hematopoietic differentiation. The sorted hematopoietic progenitor cells (HPCs) are then co-cultured with another stromal cell line and a cytokine cocktail containing IL-3, IL-7, IL-15, SCF, and Flt3L to generate PSC-derived NK cells. This system has been employed for both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) and has been shown to produce functional NK cells capable of cytokine production and cytolytic activity against tumor cells in vitro and in vivo.
The 3D culture system involves the formation of embryoid bodies (EBs) from PSCs cultured in suspension. These EBs spontaneously differentiate toward specific cell lineages in response to exogenous cytokines. To improve reproducibility, techniques like spin-EBs (centrifugation to produce uniform-sized EBs) and feeder-free systems using recombinant proteins like FcDLL-4 have been developed. These 3D systems avoid the use of feeder cells, making them more amenable to clinical applications and good manufacturing practices (GMP). However, further optimization is still needed to achieve pure and efficient iPSC-NK differentiation using these 3D approaches.
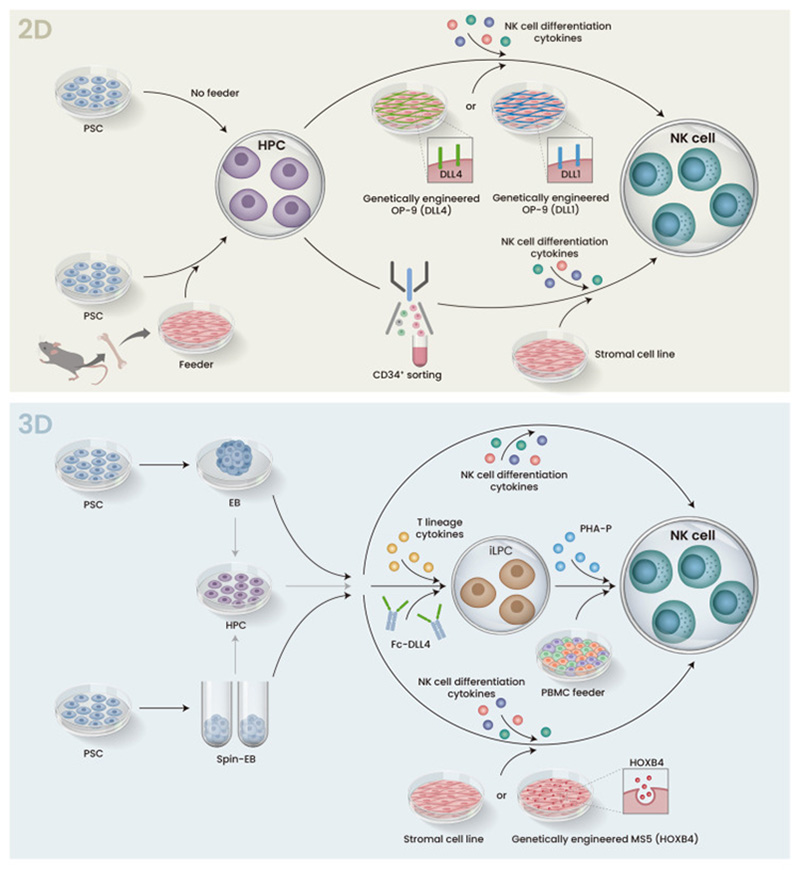
Figure 2. Schemic illustration of 2D and 3D protocol for iPSC differentiation to NK cells
Overall, researchers can leverage the advantages of both 2D and 3D platforms at different differentiation stages to optimize iPSC-NK cell production for research or therapeutic applications.
Allogeneic CAR-NK Therapy Companies
As the field of CAR-NK cell therapy continues to rapidly advance, several biotechnology companies have emerged as key players in translating this innovative approach into clinical reality. By leveraging cutting-edge technologies, these companies are pioneering the development of allogeneic, off-the-shelf CAR-NK cell products with the potential to revolutionize cancer treatment. At the forefront of this endeavor are companies like Nkarta Therapeutics, Fate Therapeutics, and Century Therapeutics, each employing unique platforms and strategies to overcome the challenges associated with manufacturing, scaling, and optimizing the therapeutic potential of CAR-NK cells.
Nkarta Therapeutics
Nkarta Therapeutics is a US-based company that is developing allogeneic, off-the-shelf NK cell-based therapies to treat cancer. Their lead products, NKX101 and NKX019, is designed to target NKG2D and CD19, which tend to be overexpressed in cancer cells. Nkarta is currently in preclinical studies for the treatment of solid tumors. The company's technology platform is designed to maximize the therapeutic effect of allogeneic NK cells through robust expansion, enhanced targeting, and extended persistence of these potent immune cells.
Fate Therapeutics
Fate Therapeutics is a pioneer in the development of programmed cellular immunotherapies for cancer and immune disorders. Their focus is on the development of off-the-shelf NK products, FT576 and FT522, derived from iPSCs. Fate Therapeutics' approach aims to revolutionize the way cancer and immune disorders are treated by leveraging the unique properties of iPSCs to produce immune cells with enhanced therapeutic potential.
Century Therapeutics
Century Therapeutics is a company that is assembling a robust portfolio of allogeneic iPSC-derived NK, γδ, and αβ T cell therapy product candidates for cancer and autoimmune and inflammatory diseases. Their proprietary Allo-Evasion™ technology is designed to avoid host rejection and potentially increase the durability of clinical responses. Enhancements in this platform have led to the generation of cells with pan-resistance to host T cell, NK, and antibody mediated rejection, optimizing and increasing the probability of success of their programs across hematology, oncology, and autoimmune and inflammatory diseases.
CAR-NK cell therapy holds significant advantages over conventional CAR-T therapies positioning them as a potentially superior modality for targeted immunotherapy. While exhibiting potent anti-tumor activity against a broad range of malignancies, CAR-NK cells demonstrate a remarkably improved safety profile with lower risks of severe toxicities like CRS, neurotoxicity, and GvHD. Leveraging the innate tumor-killing mechanisms of NK cells and the scalability of induced pluripotent stem cell technology, CAR-NK therapies offer a promising path toward developing universal, off-the-shelf products that circumvent the challenges associated with autologous CAR-T manufacturing. With their unique biological properties and the potential for better efficacy and safety, CAR-NK cell therapies may emerge as the next frontier in targeted cancer immunotherapy, surpassing the current limitations of CAR-T approaches.
We are committed to the development of high-quality cell culture reagents crucial for development of clinical stage of immune cell therapy. Employing a stringent GMP-grade quality management system, we have successfully developed an array of high-quality GMP grade growth factors, including DLL4, VEGF165, Flt-3L, SCF, IL-2, IL7, IL15 and more, specifically designed to support iPSC derived NK cell therapy development and large-scale clinical manufacturing.
1. Hang S, Wang N, Sugimura R. T, NK, then macrophages: Recent advances and challenges in adaptive immunotherapy from human pluripotent stem cells. Differentiation. 2023 Mar-Apr;130:51-57. doi: 10.1016/j.diff.2023.01.001. Epub 2023 Jan 18. PMID: 36682340.
2. Karagiannis P, Kim SI. iPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol Cells. 2021 Aug 31;44(8):541-548. doi: 10.14348/molcells.2021.0078. PMID: 34373366; PMCID: PMC8424143.
3. Shankar, K., Capitini, C.M. & Saha, K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res Ther 11, 234 (2020). https://doi.org/10.1186/s13287-020-01741-4
4. Goldenson BH, Hor P, Kaufman DS. iPSC-Derived Natural Killer Cell Therapies - Expansion and Targeting. Front Immunol. 2022 Feb 3;13:841107. doi: 10.3389/fimmu.2022.841107. PMID: 35185932; PMCID: PMC8851389.
This web search service is supported by Google Inc.
