 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CC5-M52D9 | Mouse | Mouse CCR5 Protein, Flag,His Tag (Detergent) |  |
|
|
| CC5-H52P5 | Human | Fluorescent Human CCR5 Full Length Protein (VLP) | |||
| CHEK-ATP043 | Human | HEK293/Human CCR5 Stable Cell Line | |||
| CC5-H52P3 | Human | Human CCR5 Full Length Protein (VLP) | |||
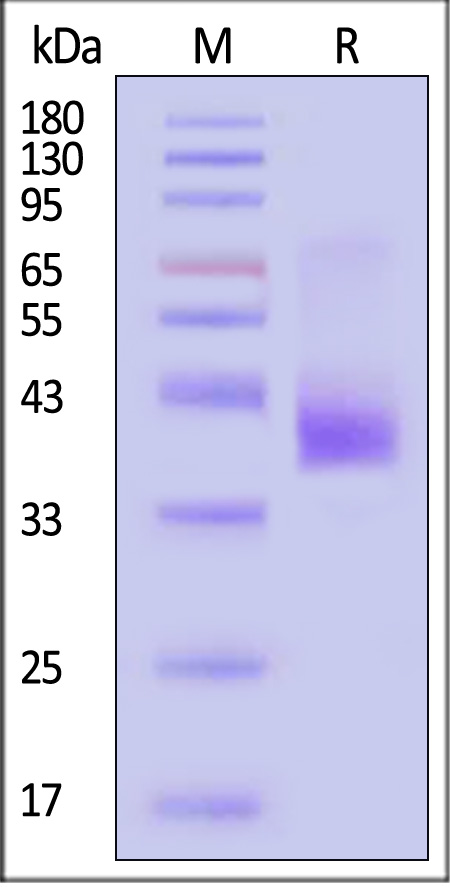
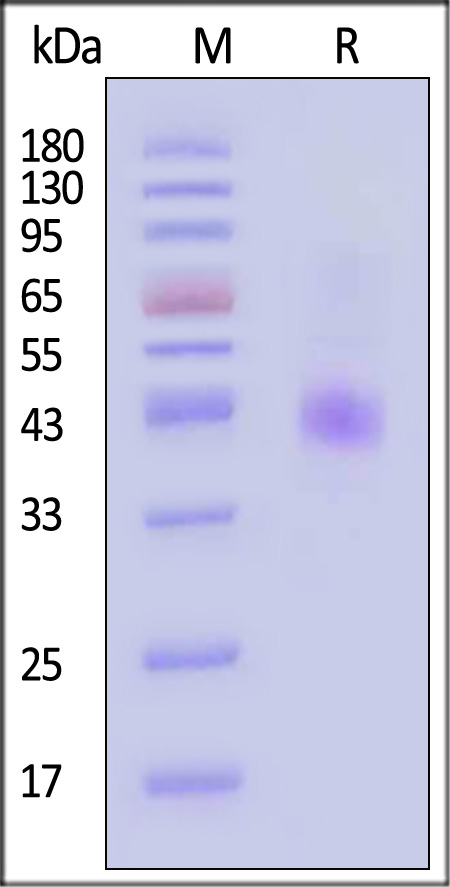
| CC5-H52D1 | Human | Human CCR5 Protein, Flag,His Tag (Detergent) |  |
|
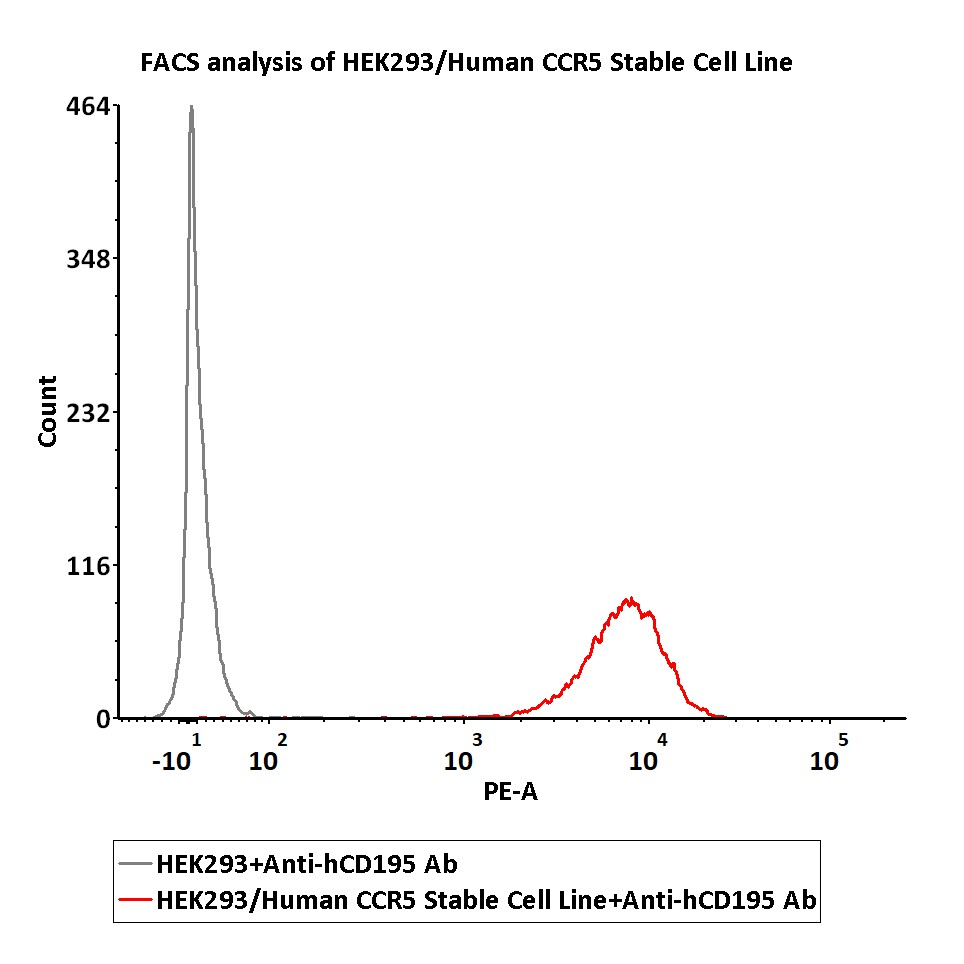
FACS assay shows that Anti-CCR5 antibody can bind to HEK293/Human CCR5 Stable Cell Line. HEK293/Human CCR5 Stable Cell Line was red line, Negative control HEK293 cells was grey line (QC tested).
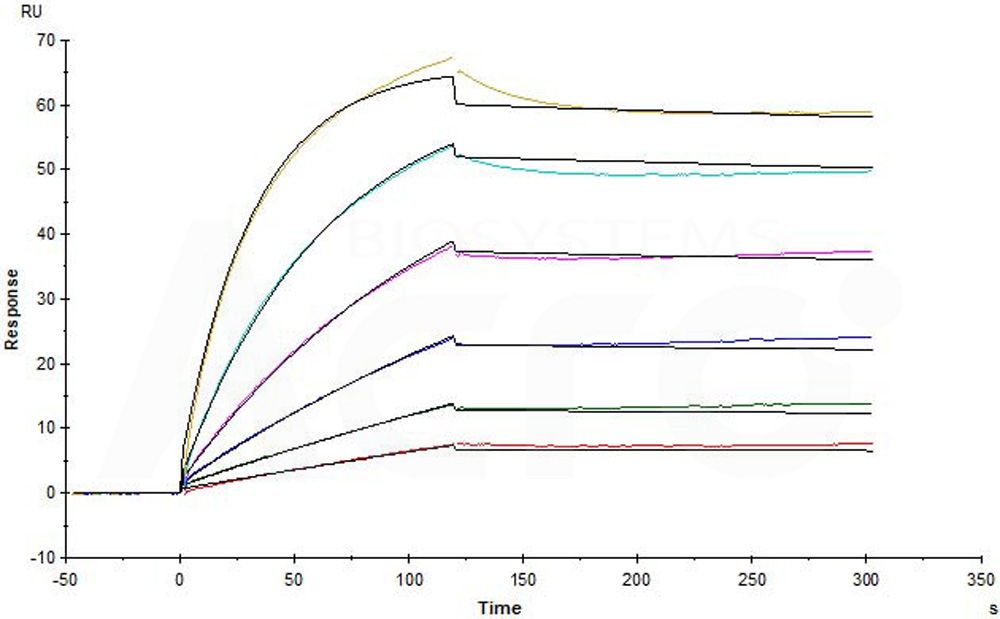
Human CCR5, Flag,His Tag (Cat. No. CC5-H52D1) captured on CM5 chip via anti-His antibody can bind Anti-CCR5 antibody (Human IgG1) with an affinity constant of 0.0737 nM as determined in a SPR assay (in presence of DDM and CHS) (Biacore T200) (Routinely tested).
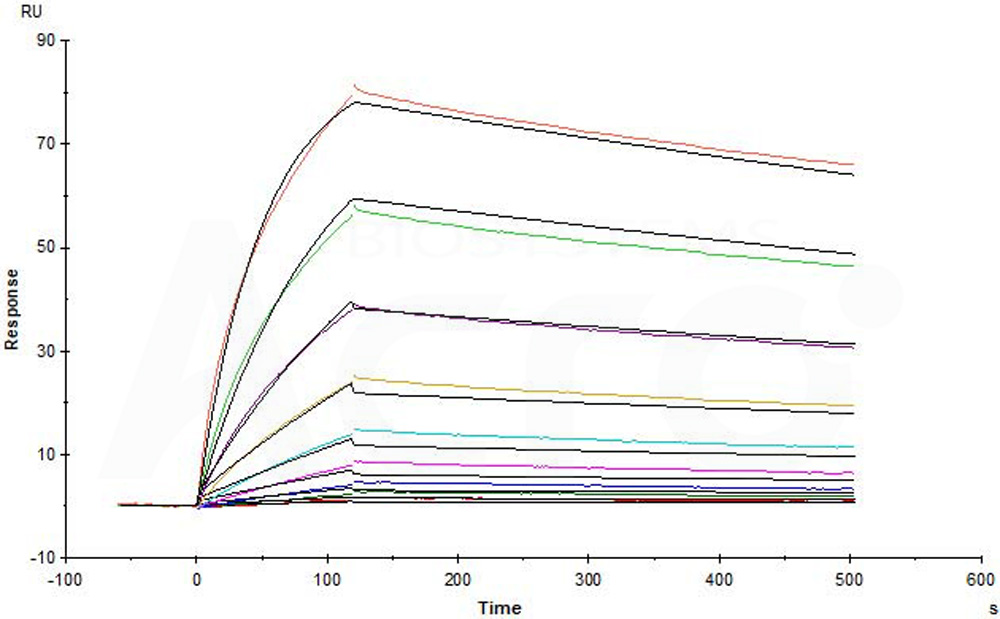
Purified NA/LE Mouse Anti-Human CD195 (Clone: 2D7) (Mouse IgG2a) captured on CM5 chip via anti-mouse antibodies surface can bind Human CCR5, Flag,His Tag (Cat. No. CC5-H52D1) with an affinity constant of 7.51 nM as determined in a SPR assay (in presence of DDM and CHS) (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Maraviroc | MVC; UK-427857 | Approved | Viiv Healthcare | Celsentri, Selzentry | United States | HIV Infections | Viiv Healthcare Co | 2007-08-06 | HIV Infections; Acquired Immunodeficiency Syndrome; Hematologic Neoplasms; Hematopoietic stem cell transplantation (HSCT); Hypertriglyceridemia; Arthritis, Rheumatoid; Graft vs Host Disease; Coronavirus Disease 2019 (COVID-19); Kidney Diseases; Stroke; AIDS Dementia Complex; Cardiovascular Diseases; Inflammation; HIV Seropositivity; Sarcoma, Kaposi | Details |
| Maraviroc | MVC; UK-427857 | Approved | Viiv Healthcare | Celsentri, Selzentry | United States | HIV Infections | Viiv Healthcare Co | 2007-08-06 | HIV Infections; Acquired Immunodeficiency Syndrome; Hematologic Neoplasms; Hematopoietic stem cell transplantation (HSCT); Hypertriglyceridemia; Arthritis, Rheumatoid; Graft vs Host Disease; Coronavirus Disease 2019 (COVID-19); Kidney Diseases; Stroke; AIDS Dementia Complex; Cardiovascular Diseases; Inflammation; HIV Seropositivity; Sarcoma, Kaposi | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Leronlimab | PRO-140; PA-14; HuPRO-140 | Phase 3 Clinical | Cytodyn Inc | HIV Infections; Solid tumours; Metabolic Dysfunction-Associated Steatotic Liver Disease; Triple Negative Breast Neoplasms; Graft vs Host Disease; Coronavirus Disease 2019 (COVID-19); Colorectal Neoplasms; HIV Seropositivity | Details |
| Cenicriviroc mesylate | CCR5/CCR2 antagonist; TBR-652; CVC; TAK-652 | Phase 3 Clinical | Tobira Therapeutics Inc, Takeda Pharmaceutical Co Ltd | Prediabetic State; HIV Infections; Acquired Immunodeficiency Syndrome; Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Liver Cirrhosis; Coronavirus Disease 2019 (COVID-19); AIDS Dementia Complex; Cholangitis, Sclerosing; Hepatic Insufficiency; Obesity; Cognition Disorders | Details |
| SB-728-T | SB-728mR-T; SB-728-T | Phase 2 Clinical | Sangamo Biosciences | HIV Infections | Details |
| Vicriviroc | SCH-D; MK-4176; SCH-417; Sch-417690 | Phase 2 Clinical | Merck & Co Inc | HIV Infections; Acquired Immunodeficiency Syndrome; Colorectal Neoplasms | Details |
| BMS-813160 | BMS-813160 | Phase 2 Clinical | Bristol-Myers Squibb Company | Pancreatic Neoplasms; Colorectal Neoplasms; Hyperplasia; Diabetic Nephropathies; Carcinoma, Pancreatic Ductal; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Cenicriviroc mesylate/Tropifexor | LJC-242 | Phase 2 Clinical | Novartis Pharma Ag | Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| AGT 103-T | AGT 103-T | Phase 1 Clinical | American Gene Technologies International Inc | HIV Infections | Details |
| Thioraviroc | Phase 1 Clinical | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | HIV Infections | Details | |
| BMS-687681 | BMS-687681 | Phase 1 Clinical | Bristol-Myers Squibb Company | Neoplasms | Details |
| SB-728-HSC | SB-728-HSC; SB-728-HSPC; SB-728mR-HSPC | Phase 1 Clinical | Sangamo Biosciences | HIV Infections; HIV Seropositivity | Details |
| LF-0376 | LF-0376 | Phase 1 Clinical | Wuxi Hillhouse biomedicine Technology Co Ltd | Solid tumours; Liver Neoplasms; Lymphoma, Large B-Cell, Diffuse; Pancreatic Neoplasms; Colorectal Neoplasms; Lymphoma | Details |
| OB-002 | OB-002; OB-002M; OB-002O; OB-002H; OB-002C; 5P12-RANTES; 5P12-RANTES-E66S | Phase 1 Clinical | Mintaka Medical Research Foundation | HIV Infections; Stomach Neoplasms; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Neoplasm Metastasis | Details |
| Leronlimab | PRO-140; PA-14; HuPRO-140 | Phase 3 Clinical | Cytodyn Inc | HIV Infections; Solid tumours; Metabolic Dysfunction-Associated Steatotic Liver Disease; Triple Negative Breast Neoplasms; Graft vs Host Disease; Coronavirus Disease 2019 (COVID-19); Colorectal Neoplasms; HIV Seropositivity | Details |
| Cenicriviroc mesylate | CCR5/CCR2 antagonist; TBR-652; CVC; TAK-652 | Phase 3 Clinical | Tobira Therapeutics Inc, Takeda Pharmaceutical Co Ltd | Prediabetic State; HIV Infections; Acquired Immunodeficiency Syndrome; Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Liver Cirrhosis; Coronavirus Disease 2019 (COVID-19); AIDS Dementia Complex; Cholangitis, Sclerosing; Hepatic Insufficiency; Obesity; Cognition Disorders | Details |
| SB-728-T | SB-728mR-T; SB-728-T | Phase 2 Clinical | Sangamo Biosciences | HIV Infections | Details |
| Vicriviroc | SCH-D; MK-4176; SCH-417; Sch-417690 | Phase 2 Clinical | Merck & Co Inc | HIV Infections; Acquired Immunodeficiency Syndrome; Colorectal Neoplasms | Details |
| BMS-813160 | BMS-813160 | Phase 2 Clinical | Bristol-Myers Squibb Company | Pancreatic Neoplasms; Colorectal Neoplasms; Hyperplasia; Diabetic Nephropathies; Carcinoma, Pancreatic Ductal; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Cenicriviroc mesylate/Tropifexor | LJC-242 | Phase 2 Clinical | Novartis Pharma Ag | Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| AGT 103-T | AGT 103-T | Phase 1 Clinical | American Gene Technologies International Inc | HIV Infections | Details |
| Thioraviroc | Phase 1 Clinical | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | HIV Infections | Details | |
| BMS-687681 | BMS-687681 | Phase 1 Clinical | Bristol-Myers Squibb Company | Neoplasms | Details |
| SB-728-HSC | SB-728-HSC; SB-728-HSPC; SB-728mR-HSPC | Phase 1 Clinical | Sangamo Biosciences | HIV Infections; HIV Seropositivity | Details |
| LF-0376 | LF-0376 | Phase 1 Clinical | Wuxi Hillhouse biomedicine Technology Co Ltd | Solid tumours; Liver Neoplasms; Lymphoma, Large B-Cell, Diffuse; Pancreatic Neoplasms; Colorectal Neoplasms; Lymphoma | Details |
| OB-002 | OB-002; OB-002M; OB-002O; OB-002H; OB-002C; 5P12-RANTES; 5P12-RANTES-E66S | Phase 1 Clinical | Mintaka Medical Research Foundation | HIV Infections; Stomach Neoplasms; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Neoplasm Metastasis | Details |
This web search service is supported by Google Inc.
