 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
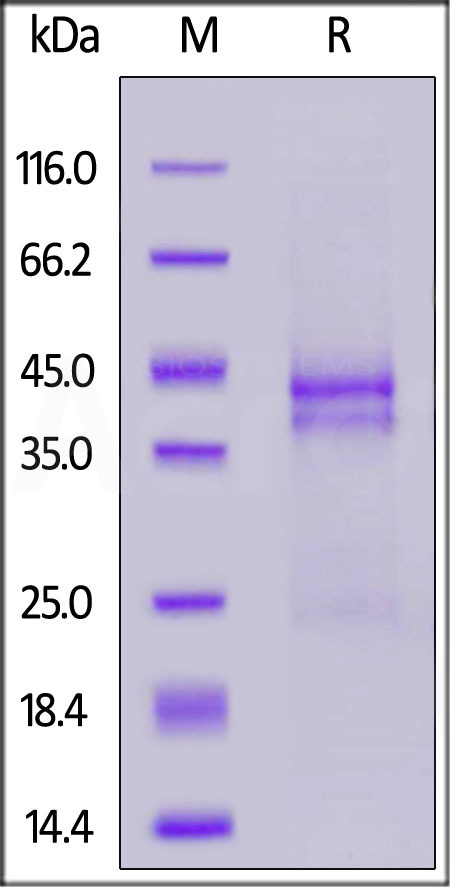
| CTF-H5113 | Human | Human CTGF / CCN2 Protein, Tag Free |  |
|
|
| GTF-H5253 | Human | Human CTGF / CCN2 Protein, Fc Tag |  |

|
|
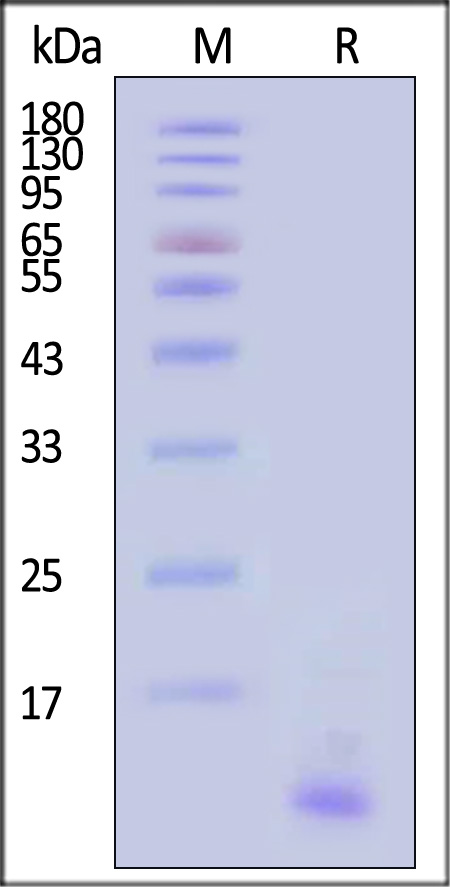
| GTF-R52H4 | Rhesus macaque | Rhesus macaque CTGF / CCN2 Protein, His Tag |  |
|
|
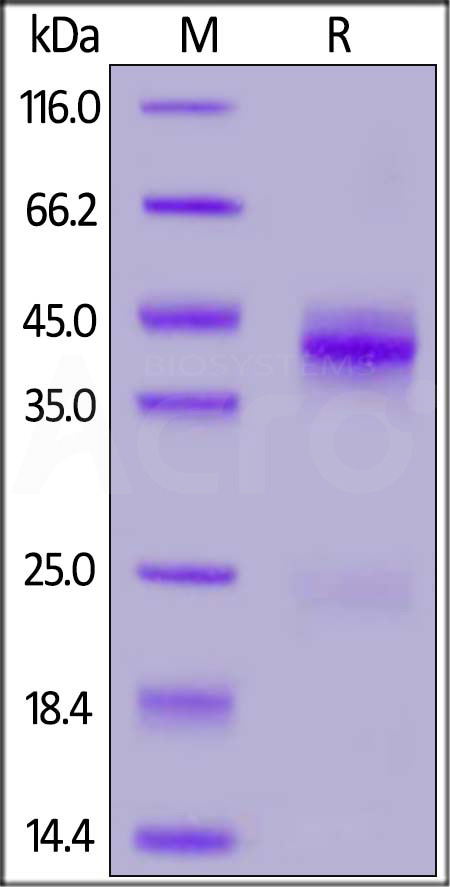
| CTF-M52Hc | Mouse | Mouse CTGF / CCN2 Protein, His Tag |  |
|
|
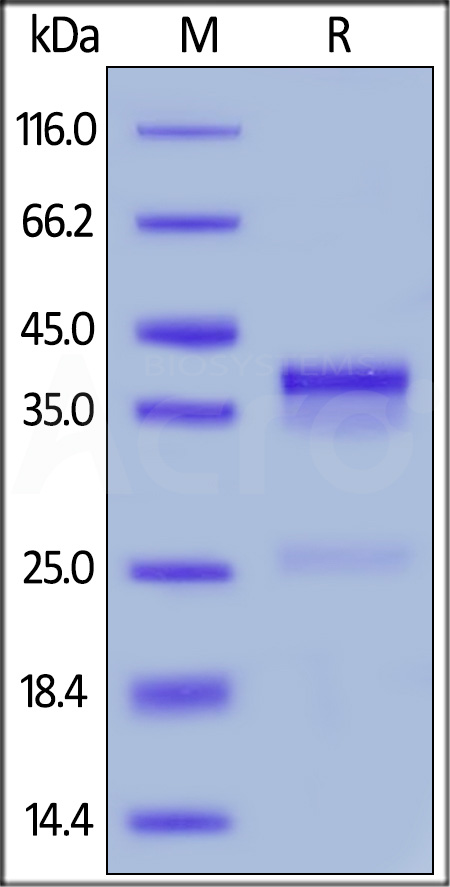
| CTF-H52H5 | Human | Human CTGF / CCN2 Protein, His Tag |  |
|
|
| CTF-H82E6 | Human | Biotinylated Human CTGF / CCN2 Protein, His,Avitag™ (recommended for biopanning) |  |
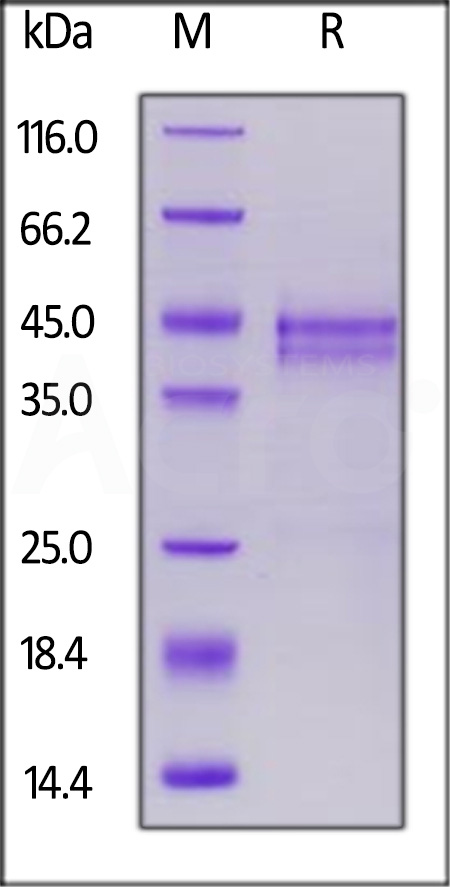
|
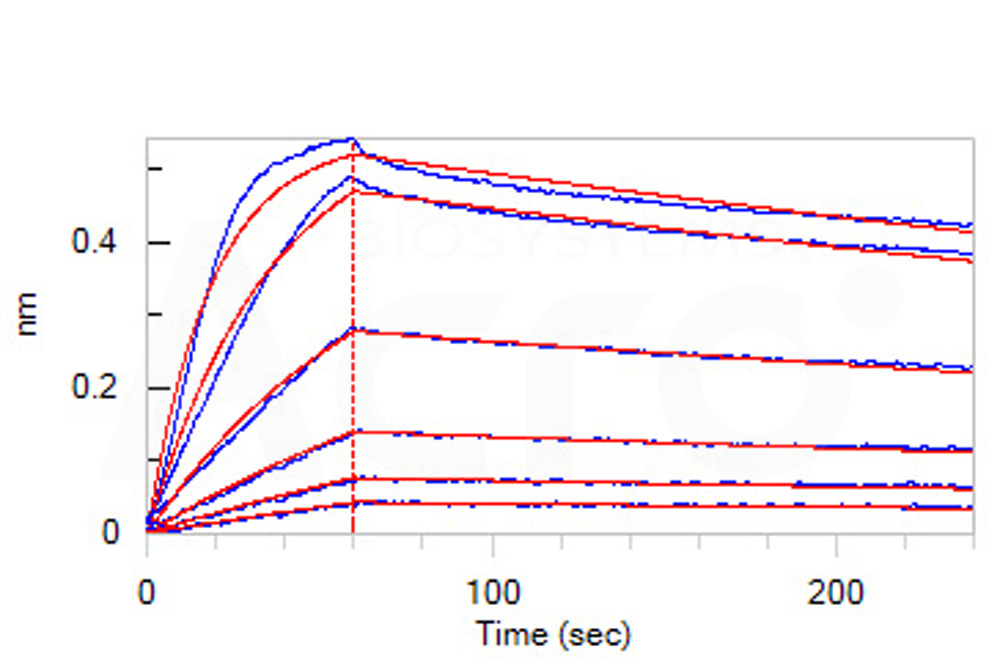
Loaded Monoclonal Anti-Human CTGF Antibody, Human IgG1 on AHC Biosensor, can bind Mouse CTGF, His Tag (Cat. No. CTF-M52Hc) with an affinity constant of 2.46 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Penicillamine | Approved | Valeant | Distamine, Pemine, Trisorcin, Cuprimine, Cupramine, Trolovol | United States | Hepatolenticular Degeneration; Cystinuria; Arthritis, Rheumatoid | Valeant Pharmaceuticals International Inc | 1970-12-04 | Heavy Metal Poisoning; Arthritis, Rheumatoid; Hepatolenticular Degeneration; Cystinuria; Lead Poisoning | Details | |
| Penicillamine | Approved | Valeant | Distamine, Pemine, Trisorcin, Cuprimine, Cupramine, Trolovol | United States | Hepatolenticular Degeneration; Cystinuria; Arthritis, Rheumatoid | Valeant Pharmaceuticals International Inc | 1970-12-04 | Heavy Metal Poisoning; Arthritis, Rheumatoid; Hepatolenticular Degeneration; Cystinuria; Lead Poisoning | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Pamrevlumab | FG-3019 | Phase 3 Clinical | Fibrogen Inc | Diabetes Mellitus, Type 2; Idiopathic Pulmonary Fibrosis; Pancreatic Neoplasms; Coronavirus Disease 2019 (COVID-19); Liver Cirrhosis; Glomerulosclerosis, Focal Segmental; Muscular Dystrophy, Duchenne; Diabetic Nephropathies; Diabetes Mellitus | Details |
| OLX-10010 | cp-asiCTGF; OLX-101A; OLX-10010; BMT-101; OLX-101 | Phase 2 Clinical | Olix Pharmaceuticals Inc | Pulmonary Fibrosis; Wet Macular Degeneration; Cicatrix, Hypertrophic; Macular Degeneration | Details |
| SHR-1906 | SHR-1906 | Phase 2 Clinical | Guangdong Hengrui Pharmaceutical Co Ltd | Idiopathic Pulmonary Fibrosis | Details |
| PRS-220 | PRS-220 | Phase 1 Clinical | Pieris Pharmaceuticals | Idiopathic Pulmonary Fibrosis | Details |
| LEM-S401 | LEM-S401; LEMS401 | Phase 1 Clinical | Lemonex Inc | Cicatrix | Details |
| Pamrevlumab | FG-3019 | Phase 3 Clinical | Fibrogen Inc | Diabetes Mellitus, Type 2; Idiopathic Pulmonary Fibrosis; Pancreatic Neoplasms; Coronavirus Disease 2019 (COVID-19); Liver Cirrhosis; Glomerulosclerosis, Focal Segmental; Muscular Dystrophy, Duchenne; Diabetic Nephropathies; Diabetes Mellitus | Details |
| OLX-10010 | cp-asiCTGF; OLX-101A; OLX-10010; BMT-101; OLX-101 | Phase 2 Clinical | Olix Pharmaceuticals Inc | Pulmonary Fibrosis; Wet Macular Degeneration; Cicatrix, Hypertrophic; Macular Degeneration | Details |
| SHR-1906 | SHR-1906 | Phase 2 Clinical | Guangdong Hengrui Pharmaceutical Co Ltd | Idiopathic Pulmonary Fibrosis | Details |
| PRS-220 | PRS-220 | Phase 1 Clinical | Pieris Pharmaceuticals | Idiopathic Pulmonary Fibrosis | Details |
| LEM-S401 | LEM-S401; LEMS401 | Phase 1 Clinical | Lemonex Inc | Cicatrix | Details |
This web search service is supported by Google Inc.
