 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
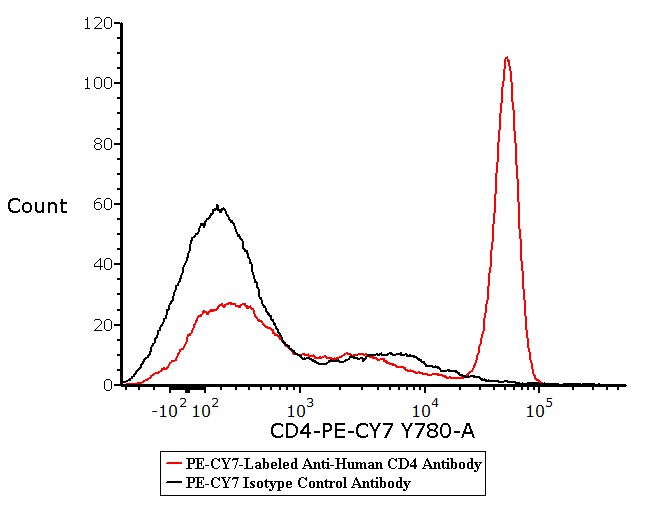
Flow cytometric analysis of Human peripheral blood lymphocytes respectively staining with PE-CY7-Labeled Anti-Human CD4 Antibody Mouse IgG1(Cat. No. FABm002-01) at 1:20 dilution (5 μL of the antibody stock solution corresponds to labeling of 2.5e5 cells in a final volume of 100 µL), compared with isotype control antibody. PE-CY7 signal was used to evaluate the binding activity (QC tested).
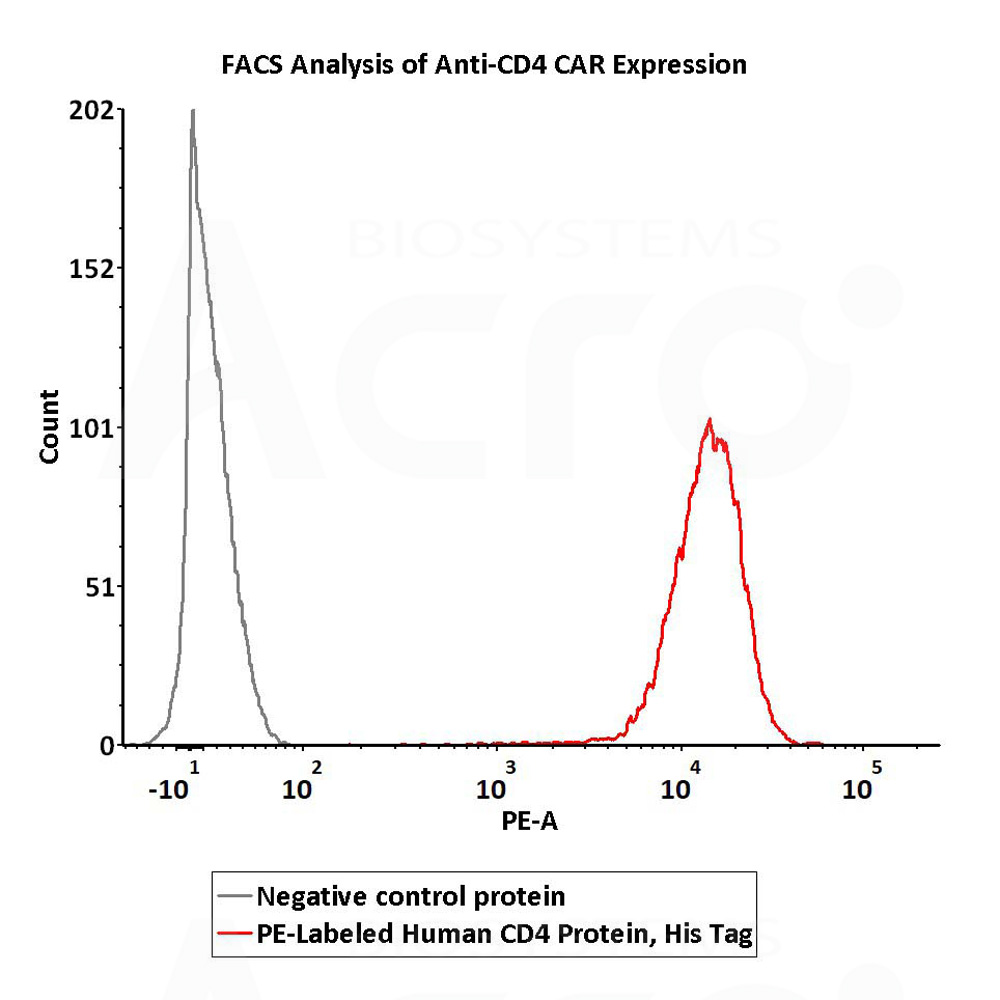
5e5 of anti-CD4 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD4, His Tag (Cat. No. CD4-HP2E3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
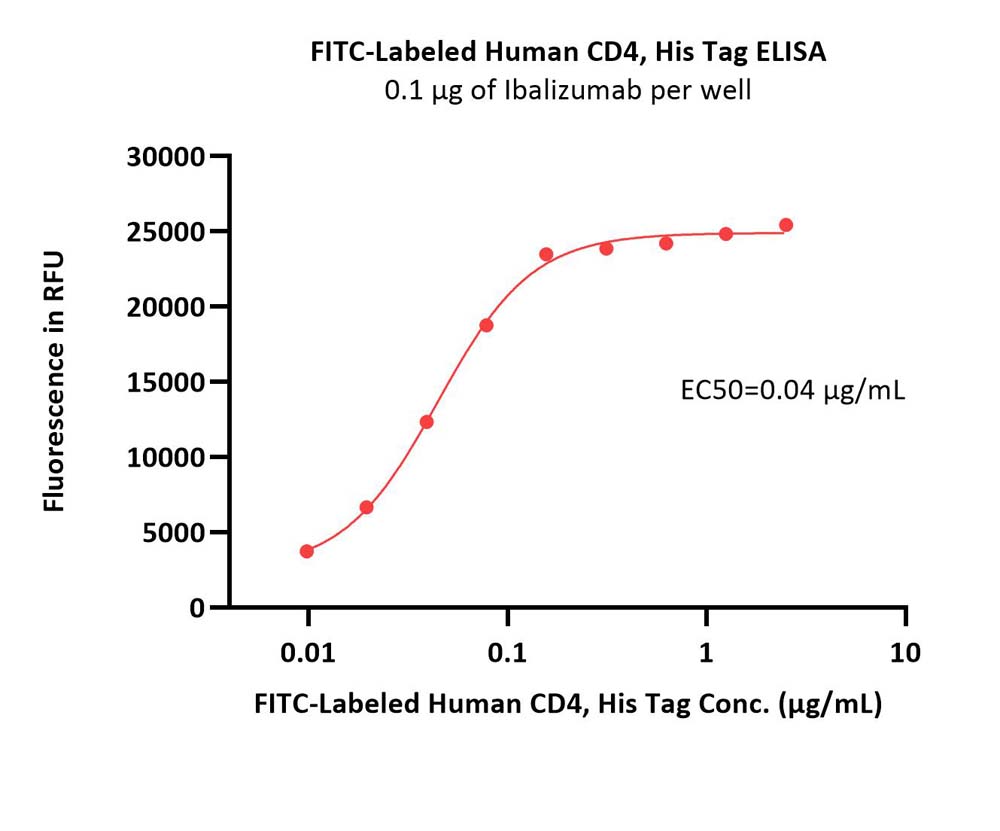
Immobilized Ibalizumab at 1 μg/mL (100 μL/well) can bind FITC-Labeled Human CD4, His Tag (Cat. No. CD4-HF2H7) with a linear range of 0.01-0.156 μg/mL (QC tested).
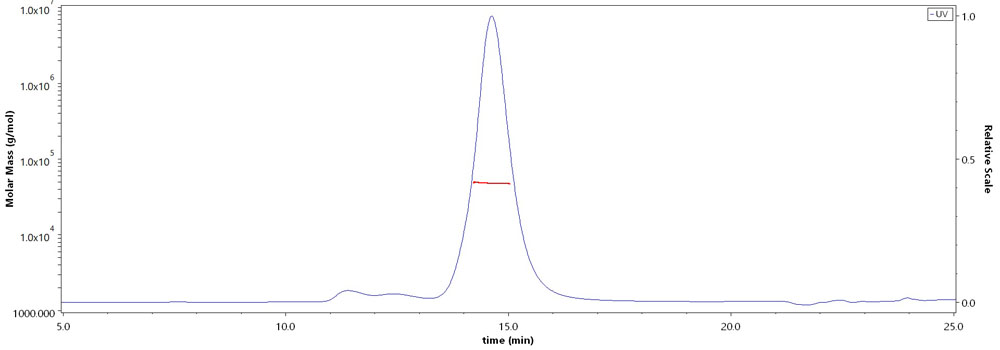
The purity of Biotinylated Rhesus macaque CD4,His,Avitag (Cat. No. CD4-R82E3) is more than 90% and the molecular weight of this protein is around 43-53 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ibalizumab | Hu5A8; TMB-355; TNX-355; 5AB | Approved | Biogen Inc | 特罗格佐, Trogarzo | United States | HIV Infections | Theratechnologies Inc | 2018-03-06 | HIV Infections | Details |
| Ibalizumab | Hu5A8; TMB-355; TNX-355; 5AB | Approved | Biogen Inc | 特罗格佐, Trogarzo | United States | HIV Infections | Theratechnologies Inc | 2018-03-06 | HIV Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Semzuvolimab | dB4; UB-421; mAb-B4 | Phase 3 Clinical | United Biomedical Inc | HIV Infections | Details |
| ITV 1(Nonindustrial source) | ITV-1 | Phase 3 Clinical | Synexa Life Sciences, Immunotech Laboratories | HIV Infections | Details |
| RB-0003 | RB-0003; BNT-111 | Phase 2 Clinical | Biontech Se, Tron | Melanoma | Details |
| VRC-01-LS | VRC-HIVMAB-080-00-AB; VRC-01-LS | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| VRC-07-523 | VRC-07-523-L S; VRC-HIVMAB075-00-AB; VRC-HIVMAB-075 -00-AB; VRC-07-523; TMB-380 | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), Taimed Biologics Inc | Acquired Immunodeficiency Syndrome; HIV Infections; Retroviridae Infections; Sexually Transmitted Diseases, Viral; HIV Seropositivity | Details |
| GEN-009 | GEN-009 | Phase 2 Clinical | Genocea Biosciences Inc | Skin Melanoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| Tregalizumab | hB-F5; BT-061 | Phase 2 Clinical | Biotest Pharma Gmbh | Drug Hypersensitivity; Arthritis, Rheumatoid; Psoriasis; Asthma | Details |
| MVA-EL | MVA-EBNA1/LMP2 | Phase 2 Clinical | Chinese University Of Hong Kong (Cuhk), Cancer Research UK | Head and Neck Neoplasms; Stomach Neoplasms; Nasopharyngeal Neoplasms; Lymphoproliferative Disorders; Epstein-Barr Virus Infections; Lymphoma | Details |
| VRC-HIVMAB091-00-AB | N6LS; Z258‐N6LS; VRC-HIVMAB091-00-AB | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| IMCY-0141 | IMCY-0141 | Phase 2 Clinical | Imcyse Sa | Multiple Sclerosis, Relapsing-Remitting | Details |
| Autologous Regulatory Т-cell Therapy | Phase 2 Clinical | Institute Of Biophysics And Cell Engineering Of National Academy Of Sciences Of Belarus | Scleroderma, Systemic | Details | |
| CD4-directed chimeric antigen receptor engineered T-cells (Icell Gene) | CD4CAR (Icell Gene) | Phase 1 Clinical | Stony Brook University School Of Medicine, Icell Gene Therapeutics (Int'L) Ltd, University Of Louisville | Leukemia, Myelomonocytic, Chronic; Lymphoma, T-Cell; Leukemia, T-Cell | Details |
| T-allo-10 | T-allo-10 | Phase 1 Clinical | Stanford University | Hematologic Diseases; Leukemia; Graft vs Host Disease; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| Anti-CD4 CAR T-cell therapy (University of Pennsylvania) | CAR-C34ZFN | Phase 1 Clinical | University Of Pennsylvania | HIV Infections; HIV Seropositivity | Details |
| 10E8.4/iMab (Aaron Diamond AIDS Research Center) | TMB-370 | Phase 1 Clinical | Aaron Diamond Aids Research Center For The City Of New York, Inc | HIV Infections | Details |
| CALRLong36 peptide (Herlev Hospital) | Phase 1 Clinical | Herlev Hospital | Myeloproliferative Disorders | Details | |
| VRC-HIVMAB060-00-AB | VRC-HIVMAB060-00-AB; VRC01 | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| VRC-HIVMAB0115-00-AB | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections; HIV Seropositivity | Details | |
| SCRI-E2CAR_EGFRtv1 | SCRI-E2CAR_EGFRtv1 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
| CD4^LVFOXP3 Treg-like cell therapy | CD4^LVFOXP3 | Phase 1 Clinical | Stanford University | Polyendocrinopathies, Autoimmune | Details |
| TMB-365 | TMB-365 | Phase 1 Clinical | Taimed Biologics Inc | HIV Infections | Details |
| LB-1901 | LB1901; LB-1901 | Phase 1 Clinical | Lymphoma, T-Cell, Peripheral; Lymphoma, T-Cell; Lymphoma, T-Cell, Cutaneous | Details | |
| IT-1208 (Kyowa Hakko Kirin) | IT-1208 | Phase 1 Clinical | Kyowa Hakko Kirin Co Ltd | Neoplasms | Details |
| Semzuvolimab | dB4; UB-421; mAb-B4 | Phase 3 Clinical | United Biomedical Inc | HIV Infections | Details |
| ITV 1(Nonindustrial source) | ITV-1 | Phase 3 Clinical | Synexa Life Sciences, Immunotech Laboratories | HIV Infections | Details |
| RB-0003 | RB-0003; BNT-111 | Phase 2 Clinical | Biontech Se, Tron | Melanoma | Details |
| VRC-01-LS | VRC-HIVMAB-080-00-AB; VRC-01-LS | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| VRC-07-523 | VRC-07-523-L S; VRC-HIVMAB075-00-AB; VRC-HIVMAB-075 -00-AB; VRC-07-523; TMB-380 | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), Taimed Biologics Inc | Acquired Immunodeficiency Syndrome; HIV Infections; Retroviridae Infections; Sexually Transmitted Diseases, Viral; HIV Seropositivity | Details |
| GEN-009 | GEN-009 | Phase 2 Clinical | Genocea Biosciences Inc | Skin Melanoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| Tregalizumab | hB-F5; BT-061 | Phase 2 Clinical | Biotest Pharma Gmbh | Drug Hypersensitivity; Arthritis, Rheumatoid; Psoriasis; Asthma | Details |
| MVA-EL | MVA-EBNA1/LMP2 | Phase 2 Clinical | Chinese University Of Hong Kong (Cuhk), Cancer Research UK | Head and Neck Neoplasms; Stomach Neoplasms; Nasopharyngeal Neoplasms; Lymphoproliferative Disorders; Epstein-Barr Virus Infections; Lymphoma | Details |
| VRC-HIVMAB091-00-AB | N6LS; Z258‐N6LS; VRC-HIVMAB091-00-AB | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| IMCY-0141 | IMCY-0141 | Phase 2 Clinical | Imcyse Sa | Multiple Sclerosis, Relapsing-Remitting | Details |
| Autologous Regulatory Т-cell Therapy | Phase 2 Clinical | Institute Of Biophysics And Cell Engineering Of National Academy Of Sciences Of Belarus | Scleroderma, Systemic | Details | |
| CD4-directed chimeric antigen receptor engineered T-cells (Icell Gene) | CD4CAR (Icell Gene) | Phase 1 Clinical | Stony Brook University School Of Medicine, Icell Gene Therapeutics (Int'L) Ltd, University Of Louisville | Leukemia, Myelomonocytic, Chronic; Lymphoma, T-Cell; Leukemia, T-Cell | Details |
| T-allo-10 | T-allo-10 | Phase 1 Clinical | Stanford University | Hematologic Diseases; Leukemia; Graft vs Host Disease; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| Anti-CD4 CAR T-cell therapy (University of Pennsylvania) | CAR-C34ZFN | Phase 1 Clinical | University Of Pennsylvania | HIV Infections; HIV Seropositivity | Details |
| 10E8.4/iMab (Aaron Diamond AIDS Research Center) | TMB-370 | Phase 1 Clinical | Aaron Diamond Aids Research Center For The City Of New York, Inc | HIV Infections | Details |
| CALRLong36 peptide (Herlev Hospital) | Phase 1 Clinical | Herlev Hospital | Myeloproliferative Disorders | Details | |
| VRC-HIVMAB060-00-AB | VRC-HIVMAB060-00-AB; VRC01 | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details |
| VRC-HIVMAB0115-00-AB | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections; HIV Seropositivity | Details | |
| SCRI-E2CAR_EGFRtv1 | SCRI-E2CAR_EGFRtv1 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
| CD4^LVFOXP3 Treg-like cell therapy | CD4^LVFOXP3 | Phase 1 Clinical | Stanford University | Polyendocrinopathies, Autoimmune | Details |
| TMB-365 | TMB-365 | Phase 1 Clinical | Taimed Biologics Inc | HIV Infections | Details |
| LB-1901 | LB1901; LB-1901 | Phase 1 Clinical | Lymphoma, T-Cell, Peripheral; Lymphoma, T-Cell; Lymphoma, T-Cell, Cutaneous | Details | |
| IT-1208 (Kyowa Hakko Kirin) | IT-1208 | Phase 1 Clinical | Kyowa Hakko Kirin Co Ltd | Neoplasms | Details |
This web search service is supported by Google Inc.
