
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
The protein has a calculated MW of 33.1 kDa & 83.1 kDa.
Virus-like particles(VLPs) are formed by self-assembly of envelop/capsid proteins from viruses. Membrane Proteins can be constituted in-situ with VLPs produced from HEK293 cell cultures. These VLPs concentrate conformationally intact membrane proteins directly on the cell surface and produce soluble, high-concentration proteins perfect for immunization and antibody screening.
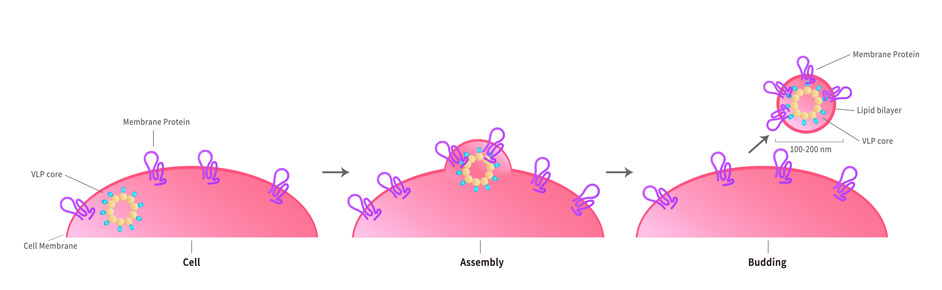
The VLPs provide the display of properly folded membrane proteins in their native cellular membrane in a compact size of 100~300 nm diameter (similar to the size of most viruses) making it optimal targets for dendritic cells in vivo and surface attachment for phage display.
The VLPs are highly immunogenic, so the immunization strategy should be optimized (antigen dose, regimen and adjuvant).
Supplied as 0.2 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
This product is supplied and shipped with dry ice, please inquire the shipping cost.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
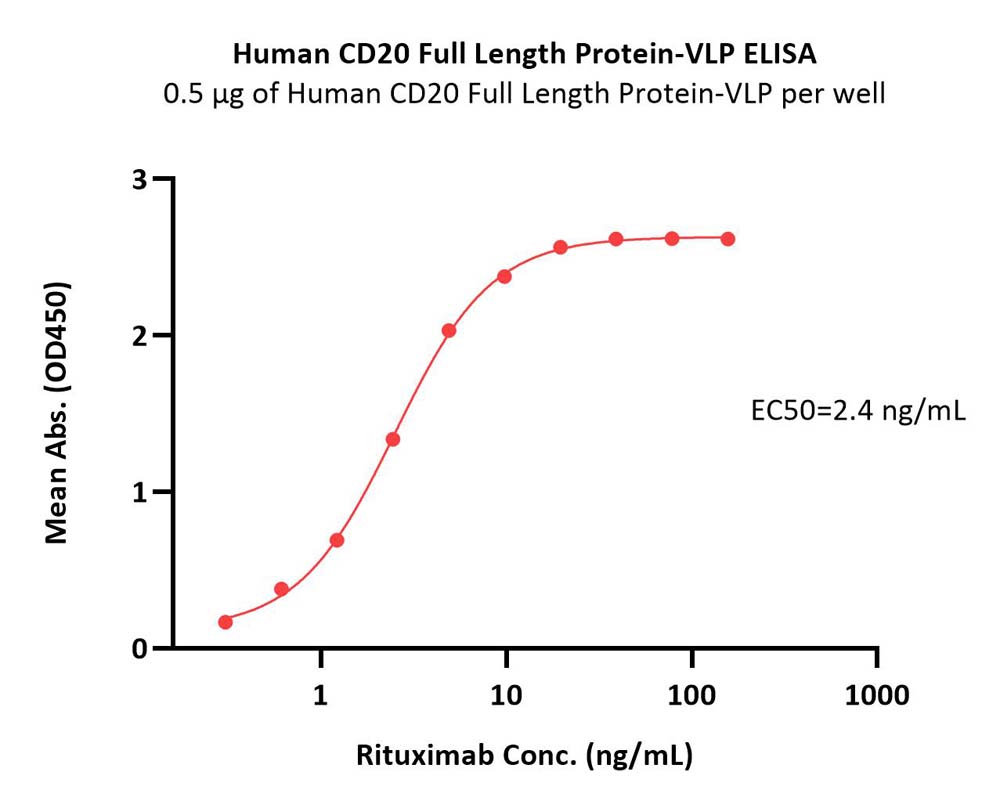
Immobilized Human CD20 Full Length Protein-VLP (Cat. No. CDP-H52P6) at 5 μg/mL (100 μL/well) can bind Rituximab biosimilar (Cat. No. CD0-M36) with a linear range of 0.02-2.5 μg/mL (QC tested).
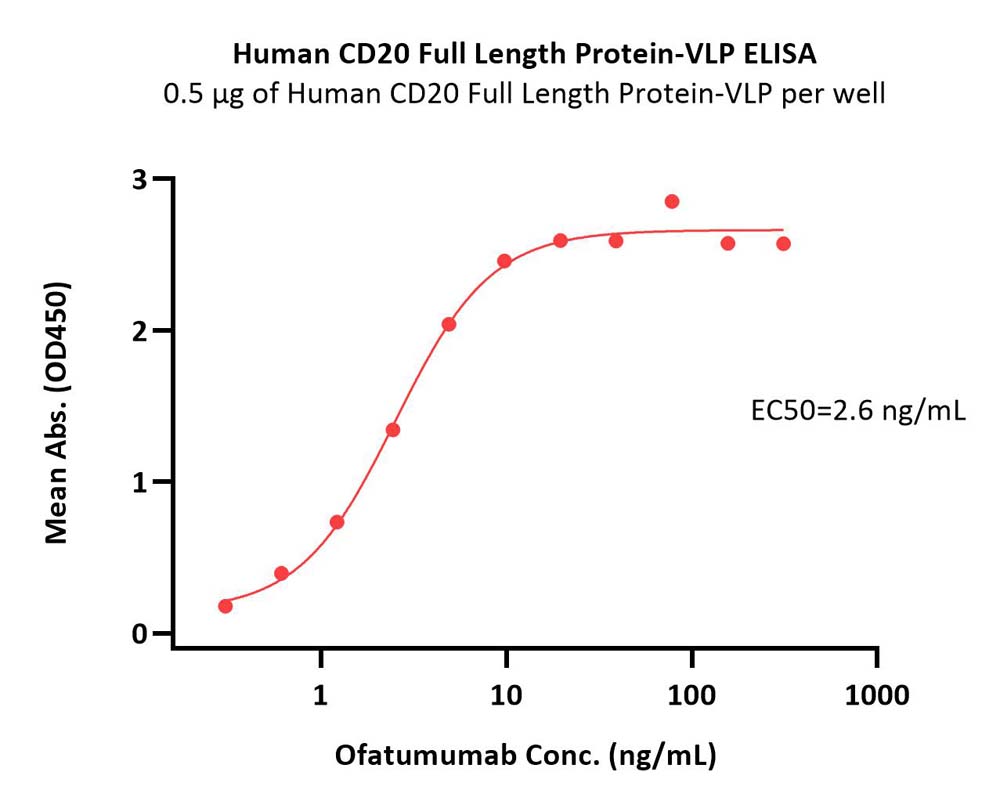
Immobilized Human CD20 Full Length Protein-VLP (Cat. No. CDP-H52P6) at 5 μg/mL (100 μL/well) can bind Ofatumumab with a linear range of 0.3-5 ng/mL (QC tested).
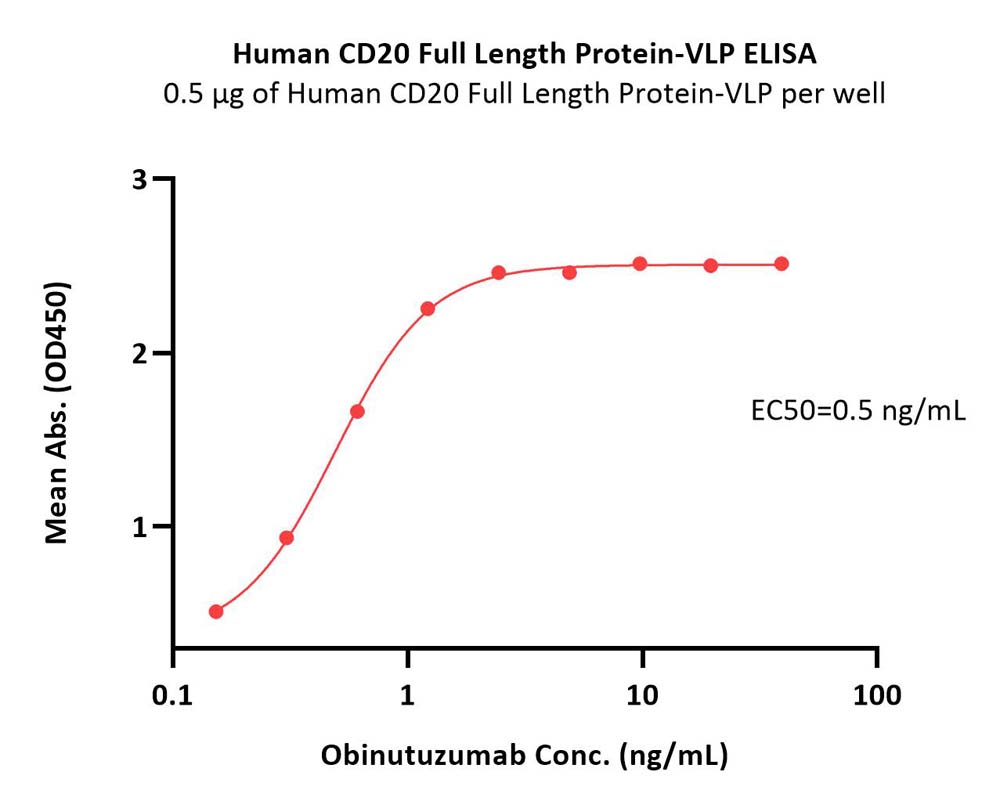
Immobilized Human CD20 Full Length Protein-VLP (Cat. No. CDP-H52P6) at 5 μg/mL (100 μL/well) can bind Obinutuzumab with a linear range of 0.2-1 ng/mL (Routinely tested).
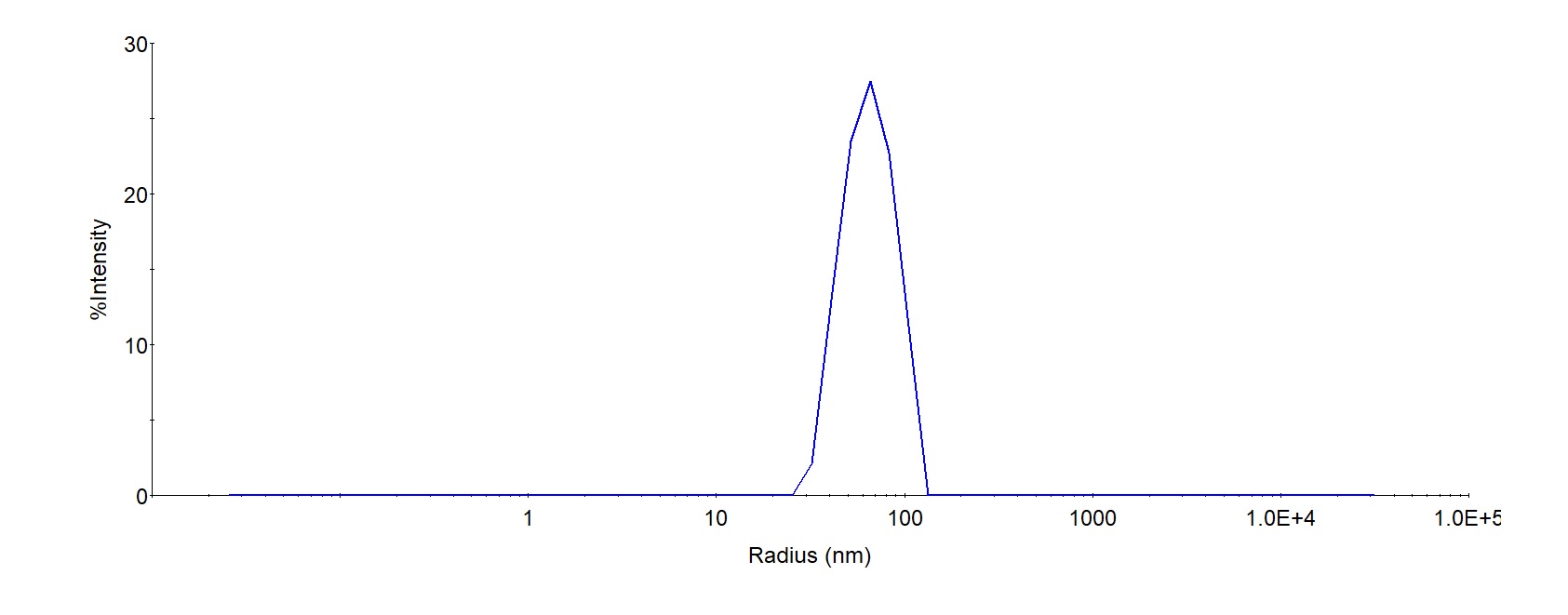
The mean peak Radius of VLP is 60-80 nm with more than 95% intensity as determined by dynamic light scattering (DLS).
Price(EUR) : €610.00
Price(EUR) : €1210.00
Price(EUR) : €4220.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Rituximab biosimilar (Celltrion) | CT-P10 | Approved | Celltrion Inc | Truxima, Blitzima, Ritemvia, Rituzena | EU | Leukemia, Lymphocytic, Chronic, B-Cell; Granulomatosis with Polyangiitis; Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid; Microscopic Polyangiitis | Celltrion Healthcare Hungary Kft | 2017-02-17 | Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Leukemia, Myelogenous, Chronic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Blast Crisis; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Anemia; Microscopic Polyangiitis; Leukemia, Lymphoid; Granulomatosis with Polyangiitis; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase | Details |
| Hyaluronidase/Rituximab | Approved | Genentech Inc | Rituxan Hycela | United States | Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Follicular | Genentech Inc | 2017-06-22 | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Ublituximab | TG-1101; TGTX-1101; LFB-R603; TG-1303; EMAB-6; R-603 | Approved | Tg Therapeutics Inc | Utuxin, BRIUMVI | United States | Multiple Sclerosis | Tg Therapeutics Inc | 2022-12-28 | Multiple Sclerosis, Relapsing-Remitting; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Sclerosis; Lymphoma, Mantle-Cell; Neuromyelitis Optica; Lymphoma, Follicular; Richter's Syndrome; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details |
| Rituximab Biosimilar(Shanghai Institute Of Biological Products) | SIBP-02 | Approved | Shanghai Institute Of Biological Products Co Ltd | 生利健 | Mainland China | Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Follicular | Shanghai Institute Of Biological Products Co Ltd | 2024-03-19 | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Ibritumomab tiuxetan | IDEC-In2B8; BAY86-5128; SHL-749; IDEC-Y2B8; IDEC-129; IDEC-2B8-MX-DTPA | Approved | Biogen Inc | Zevalin, Zavalin | United States | Lymphoma, B-Cell | Spectrum Pharmaceuticals Inc | 2002-02-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Zuberitamab | HS-006 | Approved | Zhejiang Hisun Pharmaceutical Co Ltd | 安瑞昔 | Mainland China | Lymphoma, Large B-Cell, Diffuse | BioRay Pharmaceutical Co Ltd | 2023-05-12 | Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Thrombocytopenia | Details |
| Ofatumumab | OMB-157; HuMax-CD2; GSK-1841157; 2F2 | Approved | Genmab A/S | Arzerra, Kesimpta | United States | Leukemia, Lymphocytic, Chronic, B-Cell | Novartis Pharma Ag | 2009-10-26 | Leukemia; Multiple Sclerosis, Relapsing-Remitting; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Multiple Sclerosis; Lymphoma, Follicular; Lymphoma; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell; Pemphigus | Details |
| Rituximab biosimilar (Innovent Biologics) | IBI-301 | Approved | Innovent Biologics(Suzhou) Co Ltd | 达伯华, HALPRYZA | Mainland China | Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular | Innovent Biologics(Suzhou) Co Ltd | 2020-09-30 | Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma; Lymphoma, T-Cell; Burkitt Lymphoma; Leukemia, B-Cell; Philadelphia Chromosome; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Anemia; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic | Details |
| Rituximab biosimilar (Sandoz) | GP-2013 | Approved | Sandoz | Riximyo, Rixathon | EU | Microscopic Polyangiitis; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Arthritis, Rheumatoid; Granulomatosis with Polyangiitis | Sandoz Gmbh | 2017-06-15 | Granulomatosis with Polyangiitis; Lymphoma, B-Cell; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (AryoGen Biopharma) | Approved | Aryogen Biopharma | Zytux | Iran | Leukemia, Myelogenous, Chronic; Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid; Granulomatosis with Polyangiitis; Microscopic Polyangiitis | Aryogen Biopharma | 2014-01-01 | Leukemia, Myelogenous, Chronic; Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Details | |
| Rituximab biosimilar (Biosidus) | Approved | Biosidus | Argentina | Hematologic Neoplasms | Biosidus | 2013-06-01 | Hematologic Neoplasms | Details | ||
| Rituximab biosimilar (mAbxience) | RTXM-83; mAbx-02 | Approved | Mabxience Sa | Novex | Argentina | Lymphoma, Large B-Cell, Diffuse | Mabxience Sa | 2015-01-01 | Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Lymphoma, T-Cell; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Leukemia, Myelogenous, Chronic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Blast Crisis; Lymphoma, Large B-Cell, Diffuse; Anemia; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase | Details |
| Rituximab biosimilar (Hetero Drugs) | Approved | Hetero Drugs Ltd | Maball, Mabura | India | Lymphoma, Non-Hodgkin; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Hetero Drugs Ltd | 2015-01-01 | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Rituximab biosimilar (Center of Molecular Immunology) | Approved | Center Of Molecular Immunology | CIMAbior, RituxCIM | Cuba | Lymphoma, Non-Hodgkin | null | 2017-04-01 | Lymphoma, Non-Hodgkin | Details | |
| Rituximab biosimilar (Biocad) | BCD-020 | Approved | Biocad | Acellbia | Russian Federation | Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin | Biocad | 2015-01-01 | Lymphoma, B-Cell, Marginal Zone; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (Reliance Life Sciences) | R-TPR-017 | Approved | Reliance Life Sciences | Toritz, RituxiRel | India | Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid | null | 2016-01-01 | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (CTTQ Pharma) | TQB-2303 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Mainland China | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | 2023-05-26 | Lymphoma, B-Cell; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Blastic Plasmacytoid Dendritic Cell Neoplasm; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma, T-Cell | Details | ||
| Rituximab biosimilar (Shanghai Henlius Biotech) | HLX-01 | Approved | Shanghai Henlius Biotech Inc | 汉利康 | Mainland China | Lymphoma, Non-Hodgkin | Shanghai Henlius Biopharmaceuticals Co Ltd | 2019-02-22 | Lymphoma, B-Cell; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Amgen) | ABP-798; APB-798 | Approved | Amgen Inc | Riabni | United States | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin | Amgen Inc | 2020-12-17 | Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Philadelphia Chromosome; Lymphoproliferative Disorders; Leukemia, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Plasmablastic Lymphoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hematologic Neoplasms; Blast Crisis; Blastic Plasmacytoid Dendritic Cell Neoplasm; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Anemia; Microscopic Polyangiitis; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Granulomatosis with Polyangiitis; Candidiasis, Vulvovaginal; Lymphoma, B-Cell | Details |
| Rituximab biosimilar (Pfizer) | PF-05280586; PF-5280586 | Approved | Pfizer Inc | Ruxience | United States | Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin; Microscopic Polyangiitis; Granulomatosis with Polyangiitis | Pfizer Inc | 2019-07-23 | Lymphoma, Follicular; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Philadelphia Chromosome; Leukemia, B-Cell; Lymphoproliferative Disorders; Primary mediastinal B cell lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Plasmablastic Lymphoma; Lymphoma, B-Cell; Lymphoma, Mantle-Cell; Richter's Syndrome; Blast Crisis; Lymphoma, AIDS-Related; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Microscopic Polyangiitis; Leukemia, Lymphoid; Anemia; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Granulomatosis with Polyangiitis; Lymphoma, B-Cell, Marginal Zone | Details |
| Obinutuzumab | RG-7159; RG-7195; RO-5072759; B-HH6-B-KV1-GE; R-7159; GA-101; RG7159-7 | Approved | Genentech Inc | Gazyva, Gazyvaro, Gazyva/Gazyvaro, 佳罗华 | United States | Leukemia, Lymphocytic, Chronic, B-Cell | Genentech Inc | 2013-11-01 | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Kidney Failure, Chronic; Central Nervous System Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Waldenstrom Macroglobulinemia; Lymphoma; Lymphoma, Non-Hodgkin; Nephrotic Syndrome; Lymphoproliferative Disorders; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Glomerulosclerosis, Focal Segmental; Lymphoma, Follicular; Lupus Nephritis; Glomerulonephritis, Membranous; Graft vs Host Disease; Lymphoma, Large B-Cell, Diffuse; Nephrosis; Leukemia, Lymphoid; Lymphoma, B-Cell, Marginal Zone; Leukemia | Details |
| Rituximab biosimilar (Probiomed) | PBO-326 | Approved | Probiomed | Kikuzubam | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Details | ||||
| Ripertamab | SCT-400 | Approved | SinoCelltech Ltd | Mainland China | Lymphoma, Large B-Cell, Diffuse | SinoCelltech Ltd | 2022-08-23 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Central Nervous System Lymphoma | Details | |
| Rituximab | IDEC-102; IDEC-C2B8; RO-452294; R-105; RG-105 | Approved | Biogen Inc | MabThera, MabThera/Rituxan, 美罗华, Ristova, Rituxan | United States | Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid; Leukemia, Lymphocytic, Chronic, B-Cell | Genentech Inc, Idec Pharmaceuticals Corp | 1997-11-26 | Granulomatosis with Polyangiitis; Purpura, Thrombocytopenic, Idiopathic; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Polymyositis; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Peripheral Nervous System Diseases; Rejection of renal transplantation; Non-radiographic axial spondyloarthritis; Liver Cirrhosis, Biliary; Myasthenia Gravis; Diabetes Mellitus, Type 1; HIV Infections; Leukemia; Opsoclonus-Myoclonus Syndrome; Ocular Motility Disorders; Keratoconjunctivitis Sicca; Lymphoma, B-Cell; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Multiple Sclerosis, Relapsing-Remitting; Scleritis; ST Elevation Myocardial Infarction; Microscopic Polyangiitis; Rejection of liver transplantation; Renal Insufficiency; Dermatomyositis; Nephrosis; Myositis; Schizophrenia; Hemophilia A; Immunoglobulin G4-Related Disease; Pulmonary Alveolar Proteinosis; Stomach Neoplasms; Graft vs Host Disease; Lymphoma, Large B-Cell, Diffuse; Scleroderma, Systemic; Arthritis, Rheumatoid; Kidney Diseases; P | Details |
| Ocrelizumab | R-1594; RG-1594; PRO-70769; RO-4964913; rhuMab 2H7 | Approved | Genentech Inc | Ocrevus | United States | Multiple Sclerosis | Genentech Inc | 2017-03-28 | Multiple Sclerosis, Relapsing-Remitting; Schizophrenia; Arthritis, Rheumatoid; Multiple Sclerosis; Multiple Sclerosis, Chronic Progressive; Lupus Nephritis; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin; Encephalitis | Details |
| rituximab biosimilar (Zenotech) | Approved | Zenotech Laboratories | India | Lymphoma, Non-Hodgkin | Zenotech Laboratories | 2013-02-01 | Lymphoma, Non-Hodgkin | Details | ||
| Rituximab biosimilar (Intas Biopharmaceuticals) | Approved | Intas Biopharmaceuticals | Mabtas | India | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Intas Biopharmaceuticals | 2013-01-01 | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Mosunetuzumab | BTCT-4465A; RO-7030816; CD20-TBD; RG-7828 | Approved | Genentech Inc | Lunsumio | EU | Lymphoma, Follicular | Roche Registration Gmbh | 2022-06-03 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lupus Erythematosus, Systemic; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Epcoritamab | GEN-3013; ABBV-GMAB-3013 | Approved | Genmab A/S, Abbvie Inc | Tepkinly, TEPKINLY, EPKINLY | United States | Lymphoma, Large B-Cell, Diffuse | Genmab Us Inc | 2023-05-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Richter's Syndrome; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (Dr Reddy's Laboratories) | Approved | Dr.Reddy's Laboratories Ltd | Tidecron, Reditux | India | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Dr.Reddy's Laboratories Ltd | 2007-01-01 | Arthritis, Rheumatoid; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details | |
| Glofitamab | RG-6026; RO-7082859; RG6026-2 | Approved | F. Hoffmann-La Roche Ltd | COLUMVI, 高罗华 | Canada | Lymphoma, Large B-Cell, Diffuse | F. Hoffmann-La Roche Ltd | 2023-03-25 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| Rituximab biosimilar (Celltrion) | CT-P10 | Approved | Celltrion Inc | Truxima, Blitzima, Ritemvia, Rituzena | EU | Leukemia, Lymphocytic, Chronic, B-Cell; Granulomatosis with Polyangiitis; Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid; Microscopic Polyangiitis | Celltrion Healthcare Hungary Kft | 2017-02-17 | Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Leukemia, Myelogenous, Chronic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Blast Crisis; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Anemia; Microscopic Polyangiitis; Leukemia, Lymphoid; Granulomatosis with Polyangiitis; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase | Details |
| Hyaluronidase/Rituximab | Approved | Genentech Inc | Rituxan Hycela | United States | Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Follicular | Genentech Inc | 2017-06-22 | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Ublituximab | TG-1101; TGTX-1101; LFB-R603; TG-1303; EMAB-6; R-603 | Approved | Tg Therapeutics Inc | Utuxin, BRIUMVI | United States | Multiple Sclerosis | Tg Therapeutics Inc | 2022-12-28 | Multiple Sclerosis, Relapsing-Remitting; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Sclerosis; Lymphoma, Mantle-Cell; Neuromyelitis Optica; Lymphoma, Follicular; Richter's Syndrome; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details |
| Rituximab Biosimilar(Shanghai Institute Of Biological Products) | SIBP-02 | Approved | Shanghai Institute Of Biological Products Co Ltd | 生利健 | Mainland China | Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Follicular | Shanghai Institute Of Biological Products Co Ltd | 2024-03-19 | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Ibritumomab tiuxetan | IDEC-In2B8; BAY86-5128; SHL-749; IDEC-Y2B8; IDEC-129; IDEC-2B8-MX-DTPA | Approved | Biogen Inc | Zevalin, Zavalin | United States | Lymphoma, B-Cell | Spectrum Pharmaceuticals Inc | 2002-02-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Zuberitamab | HS-006 | Approved | Zhejiang Hisun Pharmaceutical Co Ltd | 安瑞昔 | Mainland China | Lymphoma, Large B-Cell, Diffuse | BioRay Pharmaceutical Co Ltd | 2023-05-12 | Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Thrombocytopenia | Details |
| Ofatumumab | OMB-157; HuMax-CD2; GSK-1841157; 2F2 | Approved | Genmab A/S | Arzerra, Kesimpta | United States | Leukemia, Lymphocytic, Chronic, B-Cell | Novartis Pharma Ag | 2009-10-26 | Leukemia; Multiple Sclerosis, Relapsing-Remitting; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Multiple Sclerosis; Lymphoma, Follicular; Lymphoma; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell; Pemphigus | Details |
| Rituximab biosimilar (Innovent Biologics) | IBI-301 | Approved | Innovent Biologics(Suzhou) Co Ltd | 达伯华, HALPRYZA | Mainland China | Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular | Innovent Biologics(Suzhou) Co Ltd | 2020-09-30 | Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma; Lymphoma, T-Cell; Burkitt Lymphoma; Leukemia, B-Cell; Philadelphia Chromosome; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Anemia; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic | Details |
| Rituximab biosimilar (Sandoz) | GP-2013 | Approved | Sandoz | Riximyo, Rixathon | EU | Microscopic Polyangiitis; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Arthritis, Rheumatoid; Granulomatosis with Polyangiitis | Sandoz Gmbh | 2017-06-15 | Granulomatosis with Polyangiitis; Lymphoma, B-Cell; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (AryoGen Biopharma) | Approved | Aryogen Biopharma | Zytux | Iran | Leukemia, Myelogenous, Chronic; Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid; Granulomatosis with Polyangiitis; Microscopic Polyangiitis | Aryogen Biopharma | 2014-01-01 | Leukemia, Myelogenous, Chronic; Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Details | |
| Rituximab biosimilar (Biosidus) | Approved | Biosidus | Argentina | Hematologic Neoplasms | Biosidus | 2013-06-01 | Hematologic Neoplasms | Details | ||
| Rituximab biosimilar (mAbxience) | RTXM-83; mAbx-02 | Approved | Mabxience Sa | Novex | Argentina | Lymphoma, Large B-Cell, Diffuse | Mabxience Sa | 2015-01-01 | Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Lymphoma, T-Cell; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Leukemia, Myelogenous, Chronic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Blast Crisis; Lymphoma, Large B-Cell, Diffuse; Anemia; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase | Details |
| Rituximab biosimilar (Hetero Drugs) | Approved | Hetero Drugs Ltd | Maball, Mabura | India | Lymphoma, Non-Hodgkin; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Hetero Drugs Ltd | 2015-01-01 | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Rituximab biosimilar (Center of Molecular Immunology) | Approved | Center Of Molecular Immunology | CIMAbior, RituxCIM | Cuba | Lymphoma, Non-Hodgkin | null | 2017-04-01 | Lymphoma, Non-Hodgkin | Details | |
| Rituximab biosimilar (Biocad) | BCD-020 | Approved | Biocad | Acellbia | Russian Federation | Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin | Biocad | 2015-01-01 | Lymphoma, B-Cell, Marginal Zone; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (Reliance Life Sciences) | R-TPR-017 | Approved | Reliance Life Sciences | Toritz, RituxiRel | India | Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid | null | 2016-01-01 | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (CTTQ Pharma) | TQB-2303 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Mainland China | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | 2023-05-26 | Lymphoma, B-Cell; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Blastic Plasmacytoid Dendritic Cell Neoplasm; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma, T-Cell | Details | ||
| Rituximab biosimilar (Shanghai Henlius Biotech) | HLX-01 | Approved | Shanghai Henlius Biotech Inc | 汉利康 | Mainland China | Lymphoma, Non-Hodgkin | Shanghai Henlius Biopharmaceuticals Co Ltd | 2019-02-22 | Lymphoma, B-Cell; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Amgen) | ABP-798; APB-798 | Approved | Amgen Inc | Riabni | United States | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin | Amgen Inc | 2020-12-17 | Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Philadelphia Chromosome; Lymphoproliferative Disorders; Leukemia, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Plasmablastic Lymphoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hematologic Neoplasms; Blast Crisis; Blastic Plasmacytoid Dendritic Cell Neoplasm; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Anemia; Microscopic Polyangiitis; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Granulomatosis with Polyangiitis; Candidiasis, Vulvovaginal; Lymphoma, B-Cell | Details |
| Rituximab biosimilar (Pfizer) | PF-05280586; PF-5280586 | Approved | Pfizer Inc | Ruxience | United States | Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin; Microscopic Polyangiitis; Granulomatosis with Polyangiitis | Pfizer Inc | 2019-07-23 | Lymphoma, Follicular; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Philadelphia Chromosome; Leukemia, B-Cell; Lymphoproliferative Disorders; Primary mediastinal B cell lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Plasmablastic Lymphoma; Lymphoma, B-Cell; Lymphoma, Mantle-Cell; Richter's Syndrome; Blast Crisis; Lymphoma, AIDS-Related; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Microscopic Polyangiitis; Leukemia, Lymphoid; Anemia; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Granulomatosis with Polyangiitis; Lymphoma, B-Cell, Marginal Zone | Details |
| Obinutuzumab | RG-7159; RG-7195; RO-5072759; B-HH6-B-KV1-GE; R-7159; GA-101; RG7159-7 | Approved | Genentech Inc | Gazyva, Gazyvaro, Gazyva/Gazyvaro, 佳罗华 | United States | Leukemia, Lymphocytic, Chronic, B-Cell | Genentech Inc | 2013-11-01 | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Kidney Failure, Chronic; Central Nervous System Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Waldenstrom Macroglobulinemia; Lymphoma; Lymphoma, Non-Hodgkin; Nephrotic Syndrome; Lymphoproliferative Disorders; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Glomerulosclerosis, Focal Segmental; Lymphoma, Follicular; Lupus Nephritis; Glomerulonephritis, Membranous; Graft vs Host Disease; Lymphoma, Large B-Cell, Diffuse; Nephrosis; Leukemia, Lymphoid; Lymphoma, B-Cell, Marginal Zone; Leukemia | Details |
| Rituximab biosimilar (Probiomed) | PBO-326 | Approved | Probiomed | Kikuzubam | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Details | ||||
| Ripertamab | SCT-400 | Approved | SinoCelltech Ltd | Mainland China | Lymphoma, Large B-Cell, Diffuse | SinoCelltech Ltd | 2022-08-23 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Central Nervous System Lymphoma | Details | |
| Rituximab | IDEC-102; IDEC-C2B8; RO-452294; R-105; RG-105 | Approved | Biogen Inc | MabThera, MabThera/Rituxan, 美罗华, Ristova, Rituxan | United States | Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Arthritis, Rheumatoid; Leukemia, Lymphocytic, Chronic, B-Cell | Genentech Inc, Idec Pharmaceuticals Corp | 1997-11-26 | Granulomatosis with Polyangiitis; Purpura, Thrombocytopenic, Idiopathic; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Polymyositis; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Peripheral Nervous System Diseases; Rejection of renal transplantation; Non-radiographic axial spondyloarthritis; Liver Cirrhosis, Biliary; Myasthenia Gravis; Diabetes Mellitus, Type 1; HIV Infections; Leukemia; Opsoclonus-Myoclonus Syndrome; Ocular Motility Disorders; Keratoconjunctivitis Sicca; Lymphoma, B-Cell; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Multiple Sclerosis, Relapsing-Remitting; Scleritis; ST Elevation Myocardial Infarction; Microscopic Polyangiitis; Rejection of liver transplantation; Renal Insufficiency; Dermatomyositis; Nephrosis; Myositis; Schizophrenia; Hemophilia A; Immunoglobulin G4-Related Disease; Pulmonary Alveolar Proteinosis; Stomach Neoplasms; Graft vs Host Disease; Lymphoma, Large B-Cell, Diffuse; Scleroderma, Systemic; Arthritis, Rheumatoid; Kidney Diseases; P | Details |
| Ocrelizumab | R-1594; RG-1594; PRO-70769; RO-4964913; rhuMab 2H7 | Approved | Genentech Inc | Ocrevus | United States | Multiple Sclerosis | Genentech Inc | 2017-03-28 | Multiple Sclerosis, Relapsing-Remitting; Schizophrenia; Arthritis, Rheumatoid; Multiple Sclerosis; Multiple Sclerosis, Chronic Progressive; Lupus Nephritis; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin; Encephalitis | Details |
| rituximab biosimilar (Zenotech) | Approved | Zenotech Laboratories | India | Lymphoma, Non-Hodgkin | Zenotech Laboratories | 2013-02-01 | Lymphoma, Non-Hodgkin | Details | ||
| Rituximab biosimilar (Intas Biopharmaceuticals) | Approved | Intas Biopharmaceuticals | Mabtas | India | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Intas Biopharmaceuticals | 2013-01-01 | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Mosunetuzumab | BTCT-4465A; RO-7030816; CD20-TBD; RG-7828 | Approved | Genentech Inc | Lunsumio | EU | Lymphoma, Follicular | Roche Registration Gmbh | 2022-06-03 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lupus Erythematosus, Systemic; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Epcoritamab | GEN-3013; ABBV-GMAB-3013 | Approved | Genmab A/S, Abbvie Inc | Tepkinly, TEPKINLY, EPKINLY | United States | Lymphoma, Large B-Cell, Diffuse | Genmab Us Inc | 2023-05-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Richter's Syndrome; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (Dr Reddy's Laboratories) | Approved | Dr.Reddy's Laboratories Ltd | Tidecron, Reditux | India | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin | Dr.Reddy's Laboratories Ltd | 2007-01-01 | Arthritis, Rheumatoid; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details | |
| Glofitamab | RG-6026; RO-7082859; RG6026-2 | Approved | F. Hoffmann-La Roche Ltd | COLUMVI, 高罗华 | Canada | Lymphoma, Large B-Cell, Diffuse | F. Hoffmann-La Roche Ltd | 2023-03-25 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Rituximab biosimilar (Samsung) | SAIT-101 | Phase 3 Clinical | Samsung Biologics Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myelogenous, Chronic; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Arthritis, Rheumatoid; Anemia; Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase | Details |
| Rituximab biosimilar (Hualan Biological Engineering) | WBP-263 | Phase 3 Clinical | Hualan Genetic Engineering Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Boehringer Ingelheim) | BI-695500 | Phase 3 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Pancytopenia; Plasmablastic Lymphoma; Lymphoma, Mantle-Cell; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Leukemia, Myelomonocytic, Chronic; Arthritis, Rheumatoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Anemia; Candidiasis, Vulvovaginal; Hematologic Neoplasms; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Lymphoma, B-Cell, Marginal Zone | Details |
| Recombinant humanized monoclonal antibody MIL62 | MIL62 | Phase 3 Clinical | Beijing Innocare Pharma Tech Co Ltd, Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lupus Nephritis; Lupus Erythematosus, Systemic; Lymphoma, Follicular; Neuromyelitis Optica; Lymphoma, Non-Hodgkin | Details |
| Recombinant chimeric anti-CD20 antibody (Shanghai Institute of Biological Products) | Phase 3 Clinical | Shanghai Institute Of Biological Products Co Ltd | Lymphoma, Large B-Cell, Diffuse | Details | |
| Divozilimab | BCD-132 | Phase 3 Clinical | Biocad | Scleroderma, Systemic; Multiple Sclerosis; Neuromyelitis Optica | Details |
| Ocrelizumab biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Multiple Sclerosis | Details | |
| BAT-4406F | BAT-4406; BAT-4406F | Phase 3 Clinical | Bio-Thera Solutions Ltd | Neuromyelitis Optica | Details |
| Rituximab biosimilar (Nanjing Yoko Biomedical) | GB-241 | Phase 3 Clinical | Genor Biopharma Co Ltd, Nanjing Yoko Biomedical Co Ltd | Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Philadelphia Chromosome; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myelogenous, Chronic; Leukemia, Myeloid, Accelerated Phase | Details |
| Rituximab biosimilar (Hisun Pharma/Beijing Mabworks Biotech) | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd, Beijing Mabworks Biotech Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details | |
| Ocrelizumab biosimilar (Celltrion) | CT-P53 | Phase 3 Clinical | Celltrion Inc | Multiple Sclerosis, Relapsing-Remitting | Details |
| Iodine 131 tositumomab | TST/I131-TST | Phase 3 Clinical | Glaxosmithkline Plc | Lymphoma, B-Cell; Leukemia, Lymphoid; Hodgkin Disease; Multiple Myeloma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Crosslink Pharmaceutical) | B-007 | Phase 3 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd | Myasthenia Gravis; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lymphoma, Non-Hodgkin; Pemphigus | Details |
| Rituximab biosimilar(Shandong New Time Pharmaceutical) | H-02; F-007 | Phase 3 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, Large B-Cell, Diffuse | Details |
| Imvotamab | IGM-2323 | Phase 2 Clinical | Igm Biosciences Inc | Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin | Details |
| Anti-CD20 CART-transduced T cells (Cellular Biomedicine Group) | CART-20; CBM-C20.1; CBM-CD20 1 | Phase 2 Clinical | Pla General Hospital | Leukemia; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic | Details |
| BVX20-CD20 antibody (Biocon/Vaccinex) | BVX-20; BVX20-MAb | Phase 2 Clinical | Biocon Ltd, Vaccinex Inc | Lymphoma, Non-Hodgkin | Details |
| Dual specificity CD19 and CD20 or CD22 CAR-T cell therapy(Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| Anti-CD20 CAR T-cell therapy (Southwest Hospital Chongqing) | Phase 2 Clinical | Southwest Hospital Chongqing | Lymphoma, Large B-Cell, Diffuse | Details | |
| Rituximab biosimilar (JHL Biotech) | JHL-1101 | Phase 2 Clinical | JHL Biotech | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Blastic Plasmacytoid Dendritic Cell Neoplasm; Lymphoma, Follicular; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell | Details |
| Zamtocabtagene autoleucel | MB-CART2019.1 | Phase 2 Clinical | Miltenyi Biotec | Lymphoma, Large B-Cell, Diffuse | Details |
| IMM-0306 | IMC-002; IMM-0306 | Phase 2 Clinical | ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Allogeneic anti-CD20 CAR-T cell therapy (Precision BioSciences) | PBCAR-20A | Phase 2 Clinical | Precision Biosciences Inc | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ELC-301 | ELC301; ELC-301 | Phase 2 Clinical | Elicera Therapeutics AB, Uppsala University | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| MBS-303 | MBS-303 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| UCART-20x22 | UCART-20x22 | Phase 2 Clinical | Cellectis Sa | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| bbT-369 | bbT-369 | Phase 2 Clinical | Bluebird Bio Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| GB-261 | GB-261 | Phase 2 Clinical | Genor Biopharma Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CHO-H01 | CHO-H01 | Phase 2 Clinical | Academia Sinica | Neoplasms; Lymphoma, Non-Hodgkin | Details |
| MB-CART19.1 (Miltenyi Biotec) | Phase 2 Clinical | Miltenyi Biotec | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR-T | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma | Details |
| MRG001 | MRG-001 | Phase 2 Clinical | Motor Neuron Disease; Lymphoma, B-Cell; Hepatitis, Alcoholic; Cytokine Release Syndrome; Respiratory Tract Diseases; Respiratory Distress Syndrome, Adult; Lymphoma, Non-Hodgkin; Respiratory Insufficiency; Amyotrophic Lateral Sclerosis | Details | |
| IMPT-514 | IMPT-514 | Phase 2 Clinical | ImmPACT Bio USA Inc | Lupus Nephritis; Lupus Erythematosus, Systemic | Details |
| IPH-6501 | IPH-6501; IPH-65 | Phase 2 Clinical | Innate Pharma SA | Neoplasms; Lymphoma, Non-Hodgkin | Details |
| IMPT-314 | IMPT-314 | Phase 2 Clinical | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD20 monoclonal antibody(The First Affiliated Hospital of Soochow University) | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Anemia, Aplastic | Details | |
| CD19/CD20 bispecific CAR-T cells (Shanghai Cellular) | Phase 2 Clinical | Shanghai Cellular Biopharmaceutical Group Ltd | Lymphoma, B-Cell | Details | |
| ALETA-001 | ALETA-001 | Phase 2 Clinical | Aleta BioTherapeutics Inc | Lymphoma, B-Cell; Leukemia; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details |
| Rituximab Biosimilar (Istituto Giannina Gaslini) | Phase 2 Clinical | Istituto Giannina Gaslini | Nephrotic Syndrome | Details | |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| ICP-B02 | CM-355; ICP-B02 | Phase 2 Clinical | Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd | Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19/CD20 dual-target CAR-T cell therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Lymphoma, B-Cell | Details | |
| Prizloncabtagene autoleucel | C-CAR039; C-CAR-039; C CAR 039; EXP-039 | Phase 2 Clinical | Cellular Biomedicine Group Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| EX-103 | EX-103; EX103 | Phase 2 Clinical | Guangzhou Excelmab Inc | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD19 and anti-CD20 CAR-T cell therapy (Medical College of Wisconsin) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details | |
| Anti-CD20 CAR T-cell therapy (Shanghai Longyao Biotechnology) | Phase 2 Clinical | Shanghai Longyao Biological Technology Co Ltd | Lymphoma, Large B-Cell, Diffuse | Details | |
| MB-106 | MB-106 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center | Lymphoma, B-Cell; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Pancreatic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| CD19/CD20 bispecific CAR-T cells (Henan Cancer Hospital) | Phase 1 Clinical | Henan Provincial Cancer Hospital | Lymphoma, B-Cell | Details | |
| Recombinant Fc-glycosylated anti-CD20 monoclonal antibody (Bio-Thera Solutions) | BAT-4306F | Phase 1 Clinical | Bio-Thera Solutions Ltd | Lymphoma, Non-Hodgkin | Details |
| TRS-005 | TRS-005 | Phase 1 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (International Biotech Center Generium) | GNR-006 | Phase 1 Clinical | International Biotech Center Generium | Lymphoma, B-Cell | Details |
| Recombinant anti-CD20 chimeric monoclonal antibody (Livzon Group) | LZM-002 | Phase 1 Clinical | Livzon Pharmaceutical Group Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| MB-CART20.1 (Miltenyi Biotec) | Phase 1 Clinical | Miltenyi Biotec | Melanoma | Details | |
| Humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (Fujian Medical University) | Phase 1 Clinical | Fujian Medical University | Lymphoma, Large B-Cell, Diffuse | Details | |
| Anti-CD20 B9E9 scFv-Streptavidin Fusion Protein (Fred Hutchinson Cancer Research Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center, National Cancer Institute | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| Rituximab biosimilar (Shanghai CP Guojian Pharmaceutical) | CMAB-304; 304R | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Fred Hutchinson Cancer Research Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center | Lymphoma, Large B-Cell, Diffuse; Multicentric Castleman's Disease (MCD); Burkitt Lymphoma; Sarcoma, Kaposi | Details | |
| Anti-CD20 allogeneic CAR-T cell therapy (Nanjing Medical University) | LUCAR-20S | Phase 1 Clinical | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Bendamustine Hydrochloride/Rituximab | Phase 1 Clinical | 1globe Biomedical Co Ltd | Lymphoma, B-Cell | Details | |
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Pharma) | B001; B-001; B001A; B001-A; B001C; B001-C | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Multiple Sclerosis; Neuromyelitis Optica; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| Rituximab biosimilar (Gedeon Richter) | RGB-03 | Phase 1 Clinical | Gedeon Richter | Arthritis, Rheumatoid | Details |
| CD20-directed CAR-T cell therapy (Tongji Hospital) | C-CAR066 | Phase 1 Clinical | Tongji Hospital, Cellular Biomedicine Group Inc, Shanghai Cellular Biopharmaceutical Group Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| JS-203 | JS-203 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Dual CD19/CD20 targeting CAR-T therapy(Poseida Therapeutics) | Phase 1 Clinical | Poseida Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD20 CAR-T cell therapy (Shanghai Longyao Biotechnology) | Phase 1 Clinical | Shanghai Longyao Biological Technology Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| KITE-363 | KITE-363 | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse | Details | |
| ACE-1831 | ACE-1831 | Phase 1 Clinical | Acepodia Biotech Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (Jonsson Comprehensive Cancer Center) | Phase 1 Clinical | Uclas Jonsson Comprehensive Cancer Center | Candidiasis, Vulvovaginal; Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| LUCAR-G39P | LUCAR-G39P | Phase 1 Clinical | Lymphoma, Non-Hodgkin | Details | |
| Allogenic Chimeric Antigen Receptor(CAR)-T Cell Therapy(Beijing Cancer Hospital) | LUCAR-20SP | Phase 1 Clinical | Beijing Cancer Hospital | Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Mabscale) | Phase 1 Clinical | Mabscale LLC | Arthritis, Rheumatoid | Details | |
| ASP-2802 | ASP2802; ASP-2802 | Phase 1 Clinical | Astellas Pharma Global Development Inc | Lymphoma, B-Cell | Details |
| JNJ-1493 | JNJ-1493 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Lymphoma, B-Cell | Details |
| C-CAR168 CAR T-cell therapy (AbelZeta) | C-CAR168 | Phase 1 Clinical | AbelZeta Pharma Inc | Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Muscular Diseases | Details |
| SCTB-35 | SCTB35; SCTB-35 | Phase 1 Clinical | SinoCelltech Ltd | Lymphoma, B-Cell | Details |
| Recombinant human anti-CD20 momoclonal antibody(Shanghai Crosslink Pharma) | Phase 1 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd | Myasthenia Gravis; Pemphigus | Details | |
| Anti-CD19 and Anti-CD20 Bicistronic Chimeric Antigen Receptor T Cells(NIH) | Phase 1 Clinical | National Cancer Institute | Lymphoma, B-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| RO-7121932 | RO7121932; RO-7121932; RO 7121932; RG-6035 | Phase 1 Clinical | Genentech Inc | Multiple Sclerosis | Details |
| TanCART19/20 | Phase 1 Clinical | Pla General Hospital | Neuromyelitis Optica | Details | |
| LCAR-AIO | LCAR-AIO; VHH CAR-T | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CAR-20-19-22 | CAR-20-19-22 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (Zhejiang Teruisi Pharmaceutical) | TRS001 | Phase 1 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Lymphoma | Details |
| QLP-31907 | QLP-31907; QLP31907; PSB-202 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ADI-001 | ADI-001 | Phase 1 Clinical | Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma | Details | |
| TQB-2825 | TQB-2825 | Phase 1 Clinical | Wuxi Biologics Co Ltd | Hematologic Neoplasms | Details |
| CD19/CD20 CAR-T Cell Therapy (PersonGen) | Phase 1 Clinical | Persongen Biotherapeutics | Details | ||
| JMT-601 | JMT-601 | Phase 1 Clinical | Shanghai Jmt-Bio Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| LY007 | LY007 | Phase 1 Clinical | Shanghai Longyao Biological Technology Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| Autologous humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (First Affiliated Hospital of Zhejiang University) | Phase 1 Clinical | First Affiliated Hospital Of Zhejiang University | Lymphoma, Large B-Cell, Diffuse | Details | |
| Plamotamab | XmAb-13676 | Phase 1 Clinical | Xencor Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CD20/CD22 dual Targeted CAR T-cell therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University, Shanghai YaKe Biotechnology Co Ltd | Hematologic Neoplasms | Details | |
| Rituximab/Paclitaxel | AR-160 | Phase 1 Clinical | Mayo Clinic | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD20 CAR-T cell therapy (Wuhan Bio-Raid) | Clinical | Wuhan BioRaid Biotechnology Co Ltd | Neoplasms | Details | |
| Rituximab biosimilar(Bioxpress) | BXT-2336 | Clinical | Bioxpress Therapeutics Sa | Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab/Bendamustine/Cytarabine | Fondazione Italiana Linfomi Onlus | Details | |||
| Rituximab biosimilar (Samsung) | SAIT-101 | Phase 3 Clinical | Samsung Biologics Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myelogenous, Chronic; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Arthritis, Rheumatoid; Anemia; Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase | Details |
| Rituximab biosimilar (Hualan Biological Engineering) | WBP-263 | Phase 3 Clinical | Hualan Genetic Engineering Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Boehringer Ingelheim) | BI-695500 | Phase 3 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Pancytopenia; Plasmablastic Lymphoma; Lymphoma, Mantle-Cell; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Leukemia, Myelomonocytic, Chronic; Arthritis, Rheumatoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Anemia; Candidiasis, Vulvovaginal; Hematologic Neoplasms; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Lymphoma, B-Cell, Marginal Zone | Details |
| Recombinant humanized monoclonal antibody MIL62 | MIL62 | Phase 3 Clinical | Beijing Innocare Pharma Tech Co Ltd, Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lupus Nephritis; Lupus Erythematosus, Systemic; Lymphoma, Follicular; Neuromyelitis Optica; Lymphoma, Non-Hodgkin | Details |
| Recombinant chimeric anti-CD20 antibody (Shanghai Institute of Biological Products) | Phase 3 Clinical | Shanghai Institute Of Biological Products Co Ltd | Lymphoma, Large B-Cell, Diffuse | Details | |
| Divozilimab | BCD-132 | Phase 3 Clinical | Biocad | Scleroderma, Systemic; Multiple Sclerosis; Neuromyelitis Optica | Details |
| Ocrelizumab biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Multiple Sclerosis | Details | |
| BAT-4406F | BAT-4406; BAT-4406F | Phase 3 Clinical | Bio-Thera Solutions Ltd | Neuromyelitis Optica | Details |
| Rituximab biosimilar (Nanjing Yoko Biomedical) | GB-241 | Phase 3 Clinical | Genor Biopharma Co Ltd, Nanjing Yoko Biomedical Co Ltd | Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Philadelphia Chromosome; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myelogenous, Chronic; Leukemia, Myeloid, Accelerated Phase | Details |
| Rituximab biosimilar (Hisun Pharma/Beijing Mabworks Biotech) | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd, Beijing Mabworks Biotech Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details | |
| Ocrelizumab biosimilar (Celltrion) | CT-P53 | Phase 3 Clinical | Celltrion Inc | Multiple Sclerosis, Relapsing-Remitting | Details |
| Iodine 131 tositumomab | TST/I131-TST | Phase 3 Clinical | Glaxosmithkline Plc | Lymphoma, B-Cell; Leukemia, Lymphoid; Hodgkin Disease; Multiple Myeloma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Crosslink Pharmaceutical) | B-007 | Phase 3 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd | Myasthenia Gravis; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lymphoma, Non-Hodgkin; Pemphigus | Details |
| Rituximab biosimilar(Shandong New Time Pharmaceutical) | H-02; F-007 | Phase 3 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, Large B-Cell, Diffuse | Details |
| Imvotamab | IGM-2323 | Phase 2 Clinical | Igm Biosciences Inc | Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin | Details |
| Anti-CD20 CART-transduced T cells (Cellular Biomedicine Group) | CART-20; CBM-C20.1; CBM-CD20 1 | Phase 2 Clinical | Pla General Hospital | Leukemia; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic | Details |
| BVX20-CD20 antibody (Biocon/Vaccinex) | BVX-20; BVX20-MAb | Phase 2 Clinical | Biocon Ltd, Vaccinex Inc | Lymphoma, Non-Hodgkin | Details |
| Dual specificity CD19 and CD20 or CD22 CAR-T cell therapy(Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| Anti-CD20 CAR T-cell therapy (Southwest Hospital Chongqing) | Phase 2 Clinical | Southwest Hospital Chongqing | Lymphoma, Large B-Cell, Diffuse | Details | |
| Rituximab biosimilar (JHL Biotech) | JHL-1101 | Phase 2 Clinical | JHL Biotech | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Blastic Plasmacytoid Dendritic Cell Neoplasm; Lymphoma, Follicular; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell | Details |
| Zamtocabtagene autoleucel | MB-CART2019.1 | Phase 2 Clinical | Miltenyi Biotec | Lymphoma, Large B-Cell, Diffuse | Details |
| IMM-0306 | IMC-002; IMM-0306 | Phase 2 Clinical | ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Allogeneic anti-CD20 CAR-T cell therapy (Precision BioSciences) | PBCAR-20A | Phase 2 Clinical | Precision Biosciences Inc | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ELC-301 | ELC301; ELC-301 | Phase 2 Clinical | Elicera Therapeutics AB, Uppsala University | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| MBS-303 | MBS-303 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| UCART-20x22 | UCART-20x22 | Phase 2 Clinical | Cellectis Sa | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| bbT-369 | bbT-369 | Phase 2 Clinical | Bluebird Bio Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| GB-261 | GB-261 | Phase 2 Clinical | Genor Biopharma Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CHO-H01 | CHO-H01 | Phase 2 Clinical | Academia Sinica | Neoplasms; Lymphoma, Non-Hodgkin | Details |
| MB-CART19.1 (Miltenyi Biotec) | Phase 2 Clinical | Miltenyi Biotec | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR-T | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma | Details |
| MRG001 | MRG-001 | Phase 2 Clinical | Motor Neuron Disease; Lymphoma, B-Cell; Hepatitis, Alcoholic; Cytokine Release Syndrome; Respiratory Tract Diseases; Respiratory Distress Syndrome, Adult; Lymphoma, Non-Hodgkin; Respiratory Insufficiency; Amyotrophic Lateral Sclerosis | Details | |
| IMPT-514 | IMPT-514 | Phase 2 Clinical | ImmPACT Bio USA Inc | Lupus Nephritis; Lupus Erythematosus, Systemic | Details |
| IPH-6501 | IPH-6501; IPH-65 | Phase 2 Clinical | Innate Pharma SA | Neoplasms; Lymphoma, Non-Hodgkin | Details |
| IMPT-314 | IMPT-314 | Phase 2 Clinical | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD20 monoclonal antibody(The First Affiliated Hospital of Soochow University) | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Anemia, Aplastic | Details | |
| CD19/CD20 bispecific CAR-T cells (Shanghai Cellular) | Phase 2 Clinical | Shanghai Cellular Biopharmaceutical Group Ltd | Lymphoma, B-Cell | Details | |
| ALETA-001 | ALETA-001 | Phase 2 Clinical | Aleta BioTherapeutics Inc | Lymphoma, B-Cell; Leukemia; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details |
| Rituximab Biosimilar (Istituto Giannina Gaslini) | Phase 2 Clinical | Istituto Giannina Gaslini | Nephrotic Syndrome | Details | |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| ICP-B02 | CM-355; ICP-B02 | Phase 2 Clinical | Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd | Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19/CD20 dual-target CAR-T cell therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Lymphoma, B-Cell | Details | |
| Prizloncabtagene autoleucel | C-CAR039; C-CAR-039; C CAR 039; EXP-039 | Phase 2 Clinical | Cellular Biomedicine Group Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| EX-103 | EX-103; EX103 | Phase 2 Clinical | Guangzhou Excelmab Inc | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD19 and anti-CD20 CAR-T cell therapy (Medical College of Wisconsin) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details | |
| Anti-CD20 CAR T-cell therapy (Shanghai Longyao Biotechnology) | Phase 2 Clinical | Shanghai Longyao Biological Technology Co Ltd | Lymphoma, Large B-Cell, Diffuse | Details | |
| MB-106 | MB-106 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center | Lymphoma, B-Cell; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Pancreatic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| CD19/CD20 bispecific CAR-T cells (Henan Cancer Hospital) | Phase 1 Clinical | Henan Provincial Cancer Hospital | Lymphoma, B-Cell | Details | |
| Recombinant Fc-glycosylated anti-CD20 monoclonal antibody (Bio-Thera Solutions) | BAT-4306F | Phase 1 Clinical | Bio-Thera Solutions Ltd | Lymphoma, Non-Hodgkin | Details |
| TRS-005 | TRS-005 | Phase 1 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (International Biotech Center Generium) | GNR-006 | Phase 1 Clinical | International Biotech Center Generium | Lymphoma, B-Cell | Details |
| Recombinant anti-CD20 chimeric monoclonal antibody (Livzon Group) | LZM-002 | Phase 1 Clinical | Livzon Pharmaceutical Group Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| MB-CART20.1 (Miltenyi Biotec) | Phase 1 Clinical | Miltenyi Biotec | Melanoma | Details | |
| Humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (Fujian Medical University) | Phase 1 Clinical | Fujian Medical University | Lymphoma, Large B-Cell, Diffuse | Details | |
| Anti-CD20 B9E9 scFv-Streptavidin Fusion Protein (Fred Hutchinson Cancer Research Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center, National Cancer Institute | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| Rituximab biosimilar (Shanghai CP Guojian Pharmaceutical) | CMAB-304; 304R | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Fred Hutchinson Cancer Research Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center | Lymphoma, Large B-Cell, Diffuse; Multicentric Castleman's Disease (MCD); Burkitt Lymphoma; Sarcoma, Kaposi | Details | |
| Anti-CD20 allogeneic CAR-T cell therapy (Nanjing Medical University) | LUCAR-20S | Phase 1 Clinical | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Bendamustine Hydrochloride/Rituximab | Phase 1 Clinical | 1globe Biomedical Co Ltd | Lymphoma, B-Cell | Details | |
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Pharma) | B001; B-001; B001A; B001-A; B001C; B001-C | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Multiple Sclerosis; Neuromyelitis Optica; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| Rituximab biosimilar (Gedeon Richter) | RGB-03 | Phase 1 Clinical | Gedeon Richter | Arthritis, Rheumatoid | Details |
| CD20-directed CAR-T cell therapy (Tongji Hospital) | C-CAR066 | Phase 1 Clinical | Tongji Hospital, Cellular Biomedicine Group Inc, Shanghai Cellular Biopharmaceutical Group Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| JS-203 | JS-203 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Dual CD19/CD20 targeting CAR-T therapy(Poseida Therapeutics) | Phase 1 Clinical | Poseida Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD20 CAR-T cell therapy (Shanghai Longyao Biotechnology) | Phase 1 Clinical | Shanghai Longyao Biological Technology Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| KITE-363 | KITE-363 | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse | Details | |
| ACE-1831 | ACE-1831 | Phase 1 Clinical | Acepodia Biotech Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (Jonsson Comprehensive Cancer Center) | Phase 1 Clinical | Uclas Jonsson Comprehensive Cancer Center | Candidiasis, Vulvovaginal; Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| LUCAR-G39P | LUCAR-G39P | Phase 1 Clinical | Lymphoma, Non-Hodgkin | Details | |
| Allogenic Chimeric Antigen Receptor(CAR)-T Cell Therapy(Beijing Cancer Hospital) | LUCAR-20SP | Phase 1 Clinical | Beijing Cancer Hospital | Lymphoma, Non-Hodgkin | Details |
| Rituximab biosimilar (Mabscale) | Phase 1 Clinical | Mabscale LLC | Arthritis, Rheumatoid | Details | |
| ASP-2802 | ASP2802; ASP-2802 | Phase 1 Clinical | Astellas Pharma Global Development Inc | Lymphoma, B-Cell | Details |
| JNJ-1493 | JNJ-1493 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Lymphoma, B-Cell | Details |
| C-CAR168 CAR T-cell therapy (AbelZeta) | C-CAR168 | Phase 1 Clinical | AbelZeta Pharma Inc | Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Muscular Diseases | Details |
| SCTB-35 | SCTB35; SCTB-35 | Phase 1 Clinical | SinoCelltech Ltd | Lymphoma, B-Cell | Details |
| Recombinant human anti-CD20 momoclonal antibody(Shanghai Crosslink Pharma) | Phase 1 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd | Myasthenia Gravis; Pemphigus | Details | |
| Anti-CD19 and Anti-CD20 Bicistronic Chimeric Antigen Receptor T Cells(NIH) | Phase 1 Clinical | National Cancer Institute | Lymphoma, B-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| RO-7121932 | RO7121932; RO-7121932; RO 7121932; RG-6035 | Phase 1 Clinical | Genentech Inc | Multiple Sclerosis | Details |
| TanCART19/20 | Phase 1 Clinical | Pla General Hospital | Neuromyelitis Optica | Details | |
| LCAR-AIO | LCAR-AIO; VHH CAR-T | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CAR-20-19-22 | CAR-20-19-22 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab biosimilar (Zhejiang Teruisi Pharmaceutical) | TRS001 | Phase 1 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Lymphoma | Details |
| QLP-31907 | QLP-31907; QLP31907; PSB-202 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ADI-001 | ADI-001 | Phase 1 Clinical | Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma | Details | |
| TQB-2825 | TQB-2825 | Phase 1 Clinical | Wuxi Biologics Co Ltd | Hematologic Neoplasms | Details |
| CD19/CD20 CAR-T Cell Therapy (PersonGen) | Phase 1 Clinical | Persongen Biotherapeutics | Details | ||
| JMT-601 | JMT-601 | Phase 1 Clinical | Shanghai Jmt-Bio Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin | Details |
| LY007 | LY007 | Phase 1 Clinical | Shanghai Longyao Biological Technology Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| Autologous humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (First Affiliated Hospital of Zhejiang University) | Phase 1 Clinical | First Affiliated Hospital Of Zhejiang University | Lymphoma, Large B-Cell, Diffuse | Details | |
| Plamotamab | XmAb-13676 | Phase 1 Clinical | Xencor Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CD20/CD22 dual Targeted CAR T-cell therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University, Shanghai YaKe Biotechnology Co Ltd | Hematologic Neoplasms | Details | |
| Rituximab/Paclitaxel | AR-160 | Phase 1 Clinical | Mayo Clinic | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD20 CAR-T cell therapy (Wuhan Bio-Raid) | Clinical | Wuhan BioRaid Biotechnology Co Ltd | Neoplasms | Details | |
| Rituximab biosimilar(Bioxpress) | BXT-2336 | Clinical | Bioxpress Therapeutics Sa | Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rituximab/Bendamustine/Cytarabine | Fondazione Italiana Linfomi Onlus | Details |
This web search service is supported by Google Inc.







