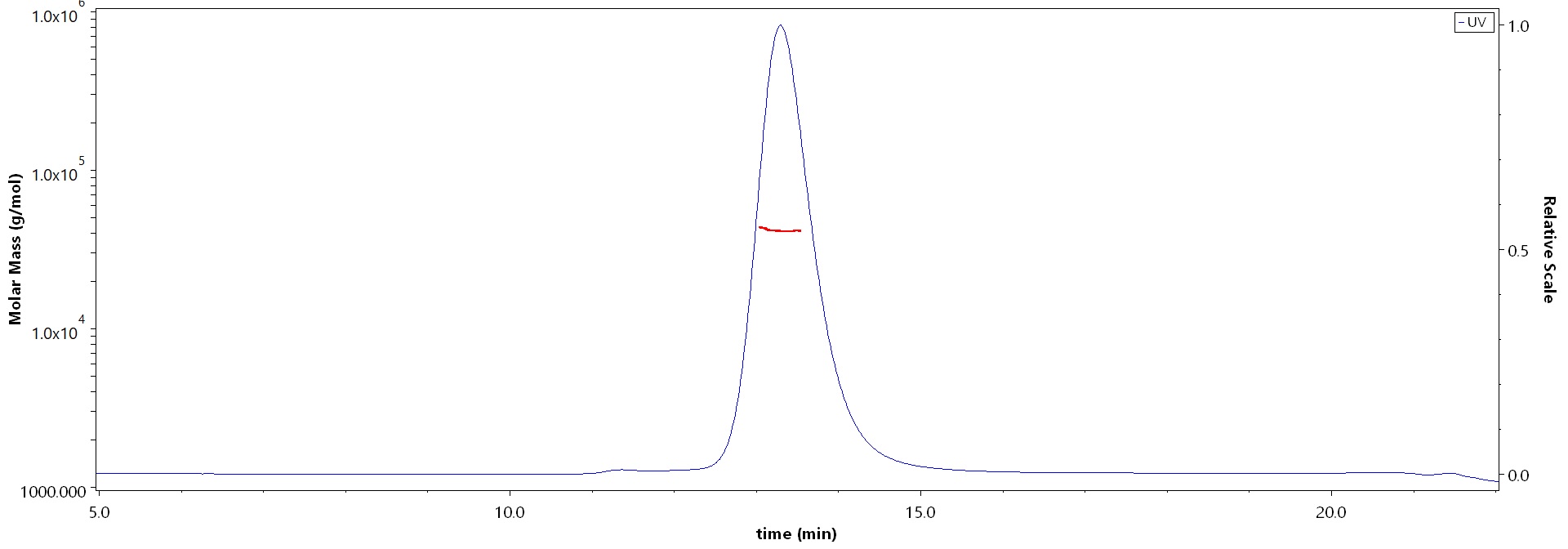
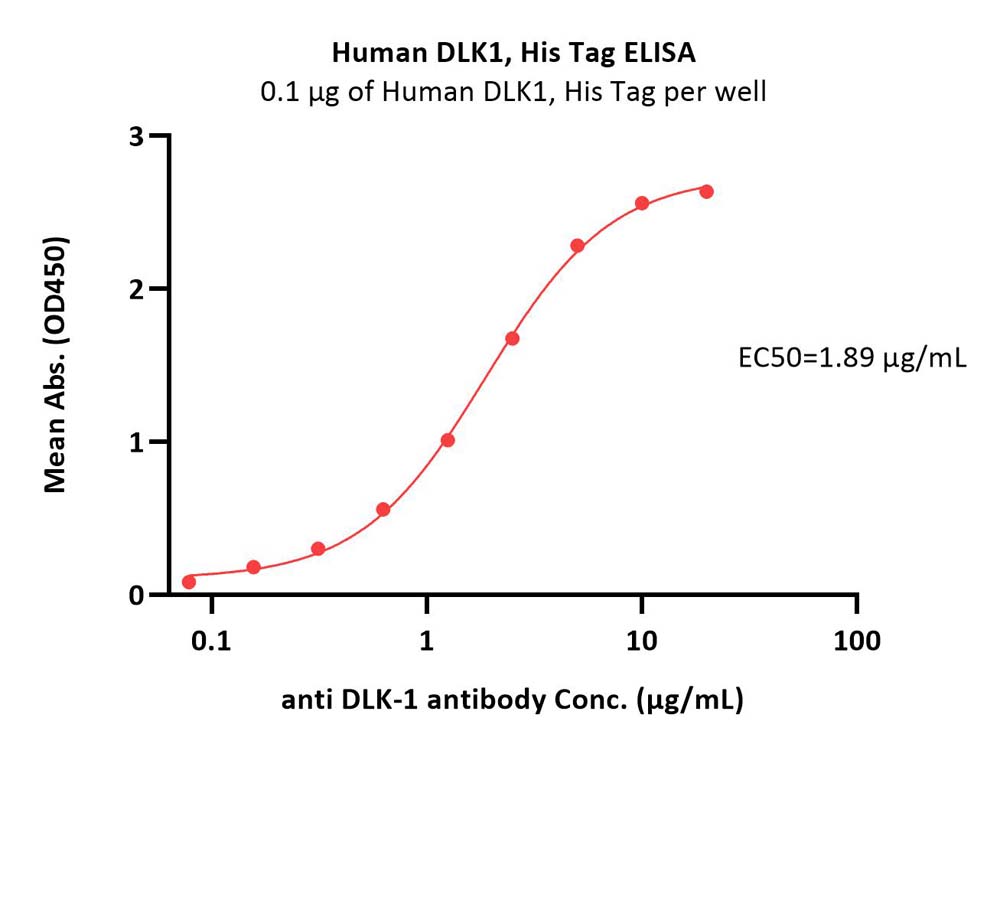
| Bamlanivimab |
LY-3819253 |
Phase 3 Clinical |
AbCellera Biologics Inc, Eli Lilly And Company |
Coronavirus Disease 2019 (COVID-19); Coronavirus Infections |
Details
|
| mRNA-1273.231 |
mRNA-1273.231 |
Phase 3 Clinical |
Moderna Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| mRNA-1273.815 |
mRNA-1273.815 |
Phase 3 Clinical |
Moderna Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Riltozinameran |
|
Phase 3 Clinical |
Biontech Se, Pfizer Inc |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| pemivibart |
VYD222; VYD-222 |
Phase 3 Clinical |
|
Coronavirus Disease 2019 (COVID-19) |
Details
|
| abdavomeran |
BNT162b1 |
Phase 3 Clinical |
Biontech Se |
Respiratory Tract Infections; RNA Virus Infections; Drug-Related Side Effects and Adverse Reactions; Virus Diseases; Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| GSK-4362620A |
GSK-4362620A |
Phase 3 Clinical |
Medicago Inc, Gsk Vaccines Gmbh |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Ogalvibart/Crexavibart |
BMS-986414 + BMS-986413 |
Phase 3 Clinical |
The Rockefeller University, Bristol Myers Squibb Srlcompany |
Coronavirus Disease 2019 (COVID-19); Coronavirus Infections |
Details
|
| Imdevimab |
RG-6412 |
Phase 3 Clinical |
F. Hoffmann-La Roche Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Simaravibart |
MAD-0004J08 |
Phase 3 Clinical |
Fondazione Toscana Life Sciences |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ABP-300 |
ABP-300; MW-05(Mabwell/Abpro) |
Phase 3 Clinical |
Mabwell (Shanghai) Bioscience Co Ltd, Abpro Corp |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Ensovibep |
MP-0420 |
Phase 3 Clinical |
Molecular Partners Ag |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| PTX-COVID19-B |
|
Phase 3 Clinical |
Providence Therapeutics Holdings Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Amubarvimab |
BRII-196; BRII196 |
Phase 3 Clinical |
Brii Biosciences (Beijing) Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Romlusevimab |
BRII198; BRII-198 |
Phase 3 Clinical |
Brii Biosciences (Beijing) Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Upanovimab |
HB-27; SCTA-01 |
Phase 3 Clinical |
SinoCelltech Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| STI-2099 |
STI-2099 |
Phase 2 Clinical |
Sorrento Therapeutics Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| SA-55 |
SA55; SA-55; BD55-5514 |
Phase 2 Clinical |
Sinovac Biotech Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Reluscovtogene ralaplasmid |
INO-4800; INO4800; PGX-9501 |
Phase 2 Clinical |
Inovio Pharmaceuticals Inc |
Coronavirus Disease 2019 (COVID-19); Coronavirus Infections; Hepatitis B |
Details
|
| SARS-CoV-2 mRNA Vaccine(Argorna) |
RBMRNA-176 |
Phase 2 Clinical |
Argorna Pharmaceuticals Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Sipavibart |
AZD-3152; AZD3152 |
Phase 2 Clinical |
Astrazeneca Plc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Recombinant SARS-CoV-2 Vaccine(variant)(Shanghai Zerun) |
|
Phase 2 Clinical |
Shanghai Zerun Biotechnology Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| HY-3000 |
HY-3000 |
Phase 2 Clinical |
Hybio Pharmaceutical Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Betuvax-CoV-2 |
|
Phase 2 Clinical |
Human Stem Cells Institute, Russia |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| FBR-002 |
FBR-002 |
Phase 2 Clinical |
Fabentech |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| IBIO123 |
IBIO-123 |
Phase 2 Clinical |
Immune Biosolutions Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Bebtelovimab |
LY-CoV1404 monoclonal antibody; LY-3853113; LY-CoV 1404 mAb; LY-CoV1404 |
Phase 2 Clinical |
Eli Lilly And Company |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| AKS-452-X |
AKS-452-X; AKS-452X |
Phase 2 Clinical |
Akston Biosciences Corp |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Casirivimab |
RG-6413 |
Phase 2 Clinical |
F. Hoffmann-La Roche Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| LY-CovMab |
LY-CovMab |
Phase 2 Clinical |
Luye Pharma Group Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| COR-101 |
STE90-C11 |
Phase 2 Clinical |
Corat Therapeutics Gmbh |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GLS-5310 |
GLS-5310 |
Phase 2 Clinical |
Geneone Life Science Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| BGB-DXP604 |
DXP-604 |
Phase 2 Clinical |
Beigene Ltd, Singlomics Biopharmaceuticals Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Lomtegovimab |
BI-767551 |
Phase 2 Clinical |
German Center For Infection Research, University Of Cologne |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| Equine immunoglobulin anti SARS-CoV-2 (Caja Costarricense de Seguro Social) |
|
Phase 2 Clinical |
Universidad De Costa Rica |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ChulaCov19 mRNA vaccine |
|
Phase 2 Clinical |
Chulalongkorn University |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| MW-33 |
MW-33; 9MW3311; 9-MW3311; 9MW-3311; 9-MW-3311 |
Phase 2 Clinical |
Mabwell (Shanghai) Bioscience Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| DXP-593 |
BGB DXP593; DXP-593 |
Phase 2 Clinical |
Singlomics Biopharmaceuticals Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Etesevimab |
LY-3832479; LY-CoV016; JS-016 |
Phase 2 Clinical |
Institute Of Microbiology Of Chinese Academy Of Sciences, Shanghai Junshi Biosciences Co Ltd |
Coronavirus Disease 2019 (COVID-19); Severe Acute Respiratory Syndrome |
Details
|
| AVACC-10 |
AVACC-10 |
Phase 1 Clinical |
Intravacc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| BAT-2022 |
BAT-2022 |
Phase 1 Clinical |
Bio-Thera Solutions Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| SCB-2020S |
SCB-2020S |
Phase 1 Clinical |
Clover Biopharmaceuticals Aus Pty Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| IN-006 |
IN-006 |
Phase 1 Clinical |
Celltrion Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| IMM-BCP-01 |
IMM-BCP-01 |
Phase 1 Clinical |
Immunome Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| RBMRNA-405 |
RBMRNA-405 |
Phase 1 Clinical |
Argorna Pharmaceuticals Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GB-0669 |
GB-0669 |
Phase 1 Clinical |
Generate Biomedicines Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| PA-001(PeptiDream) |
|
Phase 1 Clinical |
Peptidream |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| REGN17092 |
REGN-17092; REGN17092 |
Phase 1 Clinical |
|
|
Details
|
| B/HPIV3/S-6P |
B/HPIV-3/S-6-P |
Phase 1 Clinical |
National Institutes Of Health |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| F-61 |
F-61 |
Phase 1 Clinical |
Wuhan Institute Of Biological Products Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GRT-R-918 |
GRT-R918; GRT-R-918 |
Phase 1 Clinical |
Gritstone Bio Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GRT-R-914 |
GRT-R914; GRT-R-914 |
Phase 1 Clinical |
Gritstone Bio Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GRT-R-912 |
GRT-R912; GRT-R-912 |
Phase 1 Clinical |
Gritstone Bio Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| MTx-COVAB36 |
MTx-COVAB36 |
Phase 1 Clinical |
Memo Therapeutics AG |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Gorivitug |
REGN-15160 |
Phase 1 Clinical |
|
Coronavirus Disease 2019 (COVID-19) |
Details
|
| SI-F019 |
SI-F019; F-019; SZ-F019 |
Phase 1 Clinical |
|
|
Details
|
| CG-SpikeDown |
|
Phase 1 Clinical |
Caregen Co Ltd |
|
Details
|
| CT-P63 |
CT-P-63; CT-P63 |
Phase 1 Clinical |
Celltrion Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| JS-026 |
JS-026 |
Phase 1 Clinical |
Shanghai Junshi Biosciences Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ChAd-triCoV |
ChAd-triCoV |
Phase 1 Clinical |
Canadian Institutes Of Health Research (Cihr), Mcmaster University |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| Ad5-triCoV/Mac |
Ad5-triCoV/Mac |
Phase 1 Clinical |
Canadian Institutes Of Health Research (Cihr), Mcmaster University |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| JMB-2002 |
JMB-2002 |
Phase 1 Clinical |
Jiangxi Jemincare Group Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Ogalvibart |
BMS-986414 |
Phase 1 Clinical |
Bristol Myers Squibb Srlcompany, The Rockefeller University |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Crexavibart |
C144-LS; C144LS; BMS-986413 |
Phase 1 Clinical |
The Rockefeller University, Bristol Myers Squibb Srlcompany |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ABBV-47D11 |
HBM9022; ABBV-47D11; HBM-9022; 47D11 |
Phase 1 Clinical |
Abbvie Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Enuzovimab |
HFB-3013; HFB30132A |
Phase 1 Clinical |
HiFiBiO Therapeutics |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ADM-03820 |
|
Phase 1 Clinical |
Ology Bioservices Inc |
Coronavirus Disease 2019 (COVID-19); Severe Acute Respiratory Syndrome |
Details
|
| HLX-71 |
HLX-71 |
Phase 1 Clinical |
Shanghai Henlius Biotech Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Anti-SARS-CoV-2 IgY (Stanford University) |
|
Phase 1 Clinical |
Stanford University |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Bamlanivimab |
LY-3819253 |
Phase 3 Clinical |
AbCellera Biologics Inc, Eli Lilly And Company |
Coronavirus Disease 2019 (COVID-19); Coronavirus Infections |
Details
|
| mRNA-1273.231 |
mRNA-1273.231 |
Phase 3 Clinical |
Moderna Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| mRNA-1273.815 |
mRNA-1273.815 |
Phase 3 Clinical |
Moderna Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Riltozinameran |
|
Phase 3 Clinical |
Biontech Se, Pfizer Inc |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| pemivibart |
VYD222; VYD-222 |
Phase 3 Clinical |
|
Coronavirus Disease 2019 (COVID-19) |
Details
|
| abdavomeran |
BNT162b1 |
Phase 3 Clinical |
Biontech Se |
Respiratory Tract Infections; RNA Virus Infections; Drug-Related Side Effects and Adverse Reactions; Virus Diseases; Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| GSK-4362620A |
GSK-4362620A |
Phase 3 Clinical |
Medicago Inc, Gsk Vaccines Gmbh |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Ogalvibart/Crexavibart |
BMS-986414 + BMS-986413 |
Phase 3 Clinical |
The Rockefeller University, Bristol Myers Squibb Srlcompany |
Coronavirus Disease 2019 (COVID-19); Coronavirus Infections |
Details
|
| Imdevimab |
RG-6412 |
Phase 3 Clinical |
F. Hoffmann-La Roche Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Simaravibart |
MAD-0004J08 |
Phase 3 Clinical |
Fondazione Toscana Life Sciences |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ABP-300 |
ABP-300; MW-05(Mabwell/Abpro) |
Phase 3 Clinical |
Mabwell (Shanghai) Bioscience Co Ltd, Abpro Corp |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Ensovibep |
MP-0420 |
Phase 3 Clinical |
Molecular Partners Ag |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| PTX-COVID19-B |
|
Phase 3 Clinical |
Providence Therapeutics Holdings Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Amubarvimab |
BRII-196; BRII196 |
Phase 3 Clinical |
Brii Biosciences (Beijing) Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Romlusevimab |
BRII198; BRII-198 |
Phase 3 Clinical |
Brii Biosciences (Beijing) Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Upanovimab |
HB-27; SCTA-01 |
Phase 3 Clinical |
SinoCelltech Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| STI-2099 |
STI-2099 |
Phase 2 Clinical |
Sorrento Therapeutics Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| SA-55 |
SA55; SA-55; BD55-5514 |
Phase 2 Clinical |
Sinovac Biotech Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Reluscovtogene ralaplasmid |
INO-4800; INO4800; PGX-9501 |
Phase 2 Clinical |
Inovio Pharmaceuticals Inc |
Coronavirus Disease 2019 (COVID-19); Coronavirus Infections; Hepatitis B |
Details
|
| SARS-CoV-2 mRNA Vaccine(Argorna) |
RBMRNA-176 |
Phase 2 Clinical |
Argorna Pharmaceuticals Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Sipavibart |
AZD-3152; AZD3152 |
Phase 2 Clinical |
Astrazeneca Plc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Recombinant SARS-CoV-2 Vaccine(variant)(Shanghai Zerun) |
|
Phase 2 Clinical |
Shanghai Zerun Biotechnology Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| HY-3000 |
HY-3000 |
Phase 2 Clinical |
Hybio Pharmaceutical Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Betuvax-CoV-2 |
|
Phase 2 Clinical |
Human Stem Cells Institute, Russia |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| FBR-002 |
FBR-002 |
Phase 2 Clinical |
Fabentech |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| IBIO123 |
IBIO-123 |
Phase 2 Clinical |
Immune Biosolutions Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Bebtelovimab |
LY-CoV1404 monoclonal antibody; LY-3853113; LY-CoV 1404 mAb; LY-CoV1404 |
Phase 2 Clinical |
Eli Lilly And Company |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| AKS-452-X |
AKS-452-X; AKS-452X |
Phase 2 Clinical |
Akston Biosciences Corp |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Casirivimab |
RG-6413 |
Phase 2 Clinical |
F. Hoffmann-La Roche Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| LY-CovMab |
LY-CovMab |
Phase 2 Clinical |
Luye Pharma Group Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| COR-101 |
STE90-C11 |
Phase 2 Clinical |
Corat Therapeutics Gmbh |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GLS-5310 |
GLS-5310 |
Phase 2 Clinical |
Geneone Life Science Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| BGB-DXP604 |
DXP-604 |
Phase 2 Clinical |
Beigene Ltd, Singlomics Biopharmaceuticals Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Lomtegovimab |
BI-767551 |
Phase 2 Clinical |
German Center For Infection Research, University Of Cologne |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| Equine immunoglobulin anti SARS-CoV-2 (Caja Costarricense de Seguro Social) |
|
Phase 2 Clinical |
Universidad De Costa Rica |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ChulaCov19 mRNA vaccine |
|
Phase 2 Clinical |
Chulalongkorn University |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| MW-33 |
MW-33; 9MW3311; 9-MW3311; 9MW-3311; 9-MW-3311 |
Phase 2 Clinical |
Mabwell (Shanghai) Bioscience Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| DXP-593 |
BGB DXP593; DXP-593 |
Phase 2 Clinical |
Singlomics Biopharmaceuticals Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Etesevimab |
LY-3832479; LY-CoV016; JS-016 |
Phase 2 Clinical |
Institute Of Microbiology Of Chinese Academy Of Sciences, Shanghai Junshi Biosciences Co Ltd |
Coronavirus Disease 2019 (COVID-19); Severe Acute Respiratory Syndrome |
Details
|
| AVACC-10 |
AVACC-10 |
Phase 1 Clinical |
Intravacc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| BAT-2022 |
BAT-2022 |
Phase 1 Clinical |
Bio-Thera Solutions Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| SCB-2020S |
SCB-2020S |
Phase 1 Clinical |
Clover Biopharmaceuticals Aus Pty Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| IN-006 |
IN-006 |
Phase 1 Clinical |
Celltrion Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| IMM-BCP-01 |
IMM-BCP-01 |
Phase 1 Clinical |
Immunome Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| RBMRNA-405 |
RBMRNA-405 |
Phase 1 Clinical |
Argorna Pharmaceuticals Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GB-0669 |
GB-0669 |
Phase 1 Clinical |
Generate Biomedicines Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| PA-001(PeptiDream) |
|
Phase 1 Clinical |
Peptidream |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| REGN17092 |
REGN-17092; REGN17092 |
Phase 1 Clinical |
|
|
Details
|
| B/HPIV3/S-6P |
B/HPIV-3/S-6-P |
Phase 1 Clinical |
National Institutes Of Health |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| F-61 |
F-61 |
Phase 1 Clinical |
Wuhan Institute Of Biological Products Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GRT-R-918 |
GRT-R918; GRT-R-918 |
Phase 1 Clinical |
Gritstone Bio Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GRT-R-914 |
GRT-R914; GRT-R-914 |
Phase 1 Clinical |
Gritstone Bio Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| GRT-R-912 |
GRT-R912; GRT-R-912 |
Phase 1 Clinical |
Gritstone Bio Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| MTx-COVAB36 |
MTx-COVAB36 |
Phase 1 Clinical |
Memo Therapeutics AG |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Gorivitug |
REGN-15160 |
Phase 1 Clinical |
|
Coronavirus Disease 2019 (COVID-19) |
Details
|
| SI-F019 |
SI-F019; F-019; SZ-F019 |
Phase 1 Clinical |
|
|
Details
|
| CG-SpikeDown |
|
Phase 1 Clinical |
Caregen Co Ltd |
|
Details
|
| CT-P63 |
CT-P-63; CT-P63 |
Phase 1 Clinical |
Celltrion Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| JS-026 |
JS-026 |
Phase 1 Clinical |
Shanghai Junshi Biosciences Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ChAd-triCoV |
ChAd-triCoV |
Phase 1 Clinical |
Canadian Institutes Of Health Research (Cihr), Mcmaster University |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| Ad5-triCoV/Mac |
Ad5-triCoV/Mac |
Phase 1 Clinical |
Canadian Institutes Of Health Research (Cihr), Mcmaster University |
Coronavirus Disease 2019 (COVID-19); Coronaviridae Infections |
Details
|
| JMB-2002 |
JMB-2002 |
Phase 1 Clinical |
Jiangxi Jemincare Group Co Ltd |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Ogalvibart |
BMS-986414 |
Phase 1 Clinical |
Bristol Myers Squibb Srlcompany, The Rockefeller University |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Crexavibart |
C144-LS; C144LS; BMS-986413 |
Phase 1 Clinical |
The Rockefeller University, Bristol Myers Squibb Srlcompany |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ABBV-47D11 |
HBM9022; ABBV-47D11; HBM-9022; 47D11 |
Phase 1 Clinical |
Abbvie Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Enuzovimab |
HFB-3013; HFB30132A |
Phase 1 Clinical |
HiFiBiO Therapeutics |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| ADM-03820 |
|
Phase 1 Clinical |
Ology Bioservices Inc |
Coronavirus Disease 2019 (COVID-19); Severe Acute Respiratory Syndrome |
Details
|
| HLX-71 |
HLX-71 |
Phase 1 Clinical |
Shanghai Henlius Biotech Inc |
Coronavirus Disease 2019 (COVID-19) |
Details
|
| Anti-SARS-CoV-2 IgY (Stanford University) |
|
Phase 1 Clinical |
Stanford University |
Coronavirus Disease 2019 (COVID-19) |
Details
|
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now! Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions