 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > 【Inspiring Target】IL-2: from signaling pathways to therapeutic potential in tumors and autoimmune diseases Interleukin-2 (IL-2), a 15.5-kDa variably glycosylated globular protein, was the first molecule described as T cell growth factor (TCGF). It is one of the most attractive and widely studied cytokines. The ability of IL-2 to expand T cells with maintenance of functional activity has been translated into the first reproducible effective human cancer immunotherapies. Once IL-2R specifically binds to IL-2, its configuration changes, initiates JAK–STAT, PI3K–AKT–mTOR and MAPK and other pathways, inhibits apoptosis, induces cell proliferation and differentiation, and produces a variety of biological effects. With the continuous in-depth research on the signaling mechanism of IL-2 and IL-2R, the essential immune signaling will be paid more and more attention, and it is of great significance for the treatment of clinical diseases, especially tumors and autoimmune diseases.
IL-2 exerts its biological activity by acting on IL-2R on the cell membrane. IL-2R is a complex composed of IL-2Rα (CD25), IL-2Rβ (CD122) and IL-2Rγ (CD132). IL-2Rα binds to IL-2 with low affinity (Kd=10-8) and cannot conduct intracellular signal transduction. The γ subunit not only responds to IL-2, but also responds to IL-4, IL-7, IL-9, IL-15, and IL-21. When IL-2Rα, IL-2Rβ and IL-2Rγ form a trimer, the affinity is increased by 10-100 times (Kd= 10-11). IL-2Rβ and IL-2Rγ belong to the type I cytokine receptor superfamily. The γ subunit does not bind to IL-2 alone but binds to the β subunit and forms a low-affinity dimer (Kd=10-9). IL-2 binds to the above three allosteric receptors collectively referred to as a component of the IL-2 and IL-2R signal. The β and γ subunits carry signal sequences in the tails of the cytoplasm, and their signal sequences are transduced through a variety of intracellular pathways such as JAK–STAT, PI3K and MAPK.
The JAK–STAT pathway accounts for 90% of IL-2 and IL-2R signal. IL-2 binding leads to heterodimerization of IL-2Rβ and IL-2Rγ, activating the tyrosine kinases JAK1and JAK3, respectively, which phosphorylate tyrosine residues in IL-2Rβ. This promotes recruitment of signaling molecules such as PI3K, STAT5 or SHC1, which are phosphorylated by JAKs, resulting in specific pathway activation, nuclear translocation of transcription factors and finally targeted transcription regulation that induces cell activation, differentiation, and proliferation. PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2), resulting in production of phosphatidylinositol-3,4,5-trisphosphate (PIP3), which promotes recruitment of phosphoinositide-dependent kinase l (PDK1) and AKT (also known as PKB) to the cell membrane. Phosphorylation of AKT by PDK1 and mTOR complex 2 (mTORC2) is necessary for full activation. AKT phosphorylation of tuberous sclerosis complex (TSC) proteins relieve TSC-mediated inhibition of RHEB to activate mTORC1, which phosphorylates p70 ribosomal S6 kinase (p70S6K), a kinase that is important for survival, proliferation, and protein translation. Tyrosine phosphorylation of STAT5 leads to its dimerization or tetramerization, nuclear translocation and transcription activation or repression. Phosphorylation of SHC1 promotes recruitment of GRB2 and SOS, forming a complex that catalysis GTP exchange on RAS and subsequent activation of the MAPK pathway. Depending on the concentration and duration of exposure, IL-2 induces different signals in conventional T cells compared with Treg cells, which influences the outcome of a localized immune response in a pro-inflammatory setting. Aside from natural IL-2, enhanced IL-2 formulations such as muteins or IL-2–anti-IL-2 antibody complexes can be targeted to Treg cells or conventional T cells in autoimmune or cancer settings, respectively, and, depending on modified binding properties, induce stronger IL-2 signal.
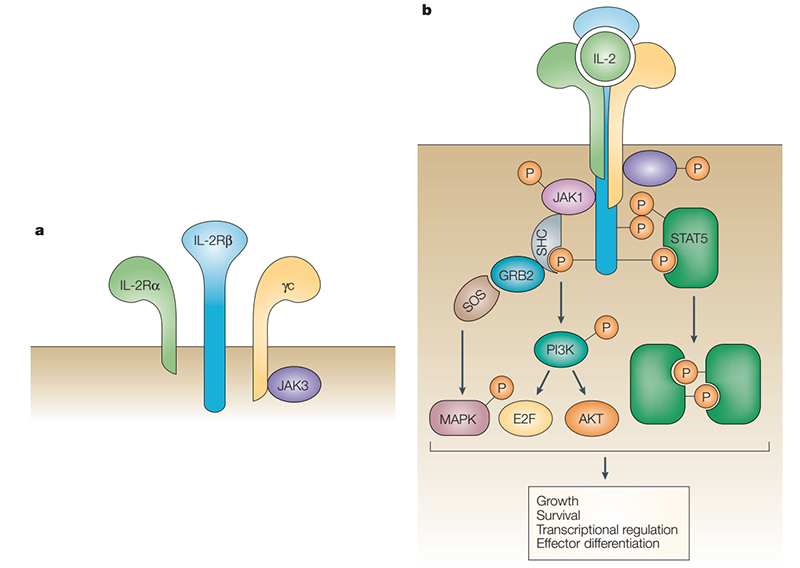
Structure and signal pathways of IL-2
IL-2 was found in the culture supernatant of activated T cells in 1976 and cloned in 1983. High doses of IL-2 seem to be important for the differentiation of effector T cells and memory effector T cells, which led to the initial development of this cytokine as an anti-cancer drug. Advanced/metastatic melanoma and renal cell carcinoma (RCC) are the two cancers in most studies evaluating the therapeutic effects of high-dose IL-2.
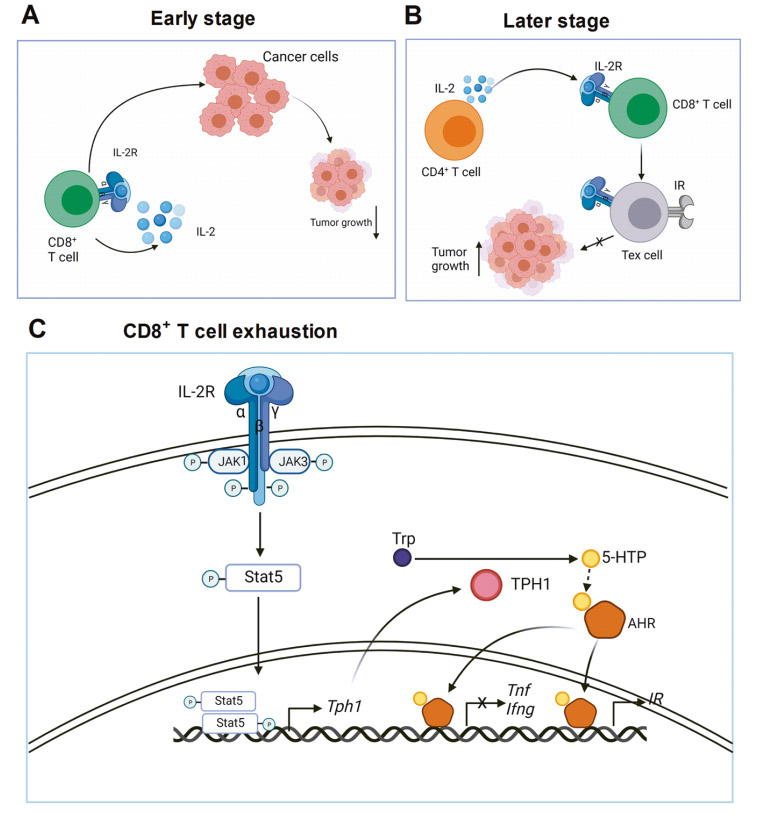
IL-2 is essential for CD8+ T cell exhaustion in tumors
In the early stage of tumor growth, IL-2 induces effector CD8+ T cell differentiation through an autocrine method. CD8+ T cells kill cancer cells and inhibit tumor growth. In the later stage of tumor growth, IL-2 continuously produced by CD4+ T cells acts on CD8+ T cells and promotes CD8+ T cell exhaustion. Therefore, tumor growth is not inhibited. IL-2 signaling pathway in CD8+ T cells phosphorylates STAT5 through JAK1 and JAK3. Phosphorylated STAT5 forms a dimer and migrates to the nucleus to transcriptionally activate TPH1 expression. The TPH1 enzyme metabolizes tryptophan to 5-HTP, and then 5-HTP binds to AHR. IL-2 induces the SUMOylation of AHR in CD8+ T cells and protects it from ubiquitination degradation. These findings have far-reaching implications for cancer immunotherapy. First, targeting IL-2 is a good way to treat advanced tumors. This can be achieved by neutralizing IL-2, blocking IL-2 receptors, or depleting IL-2 sources.
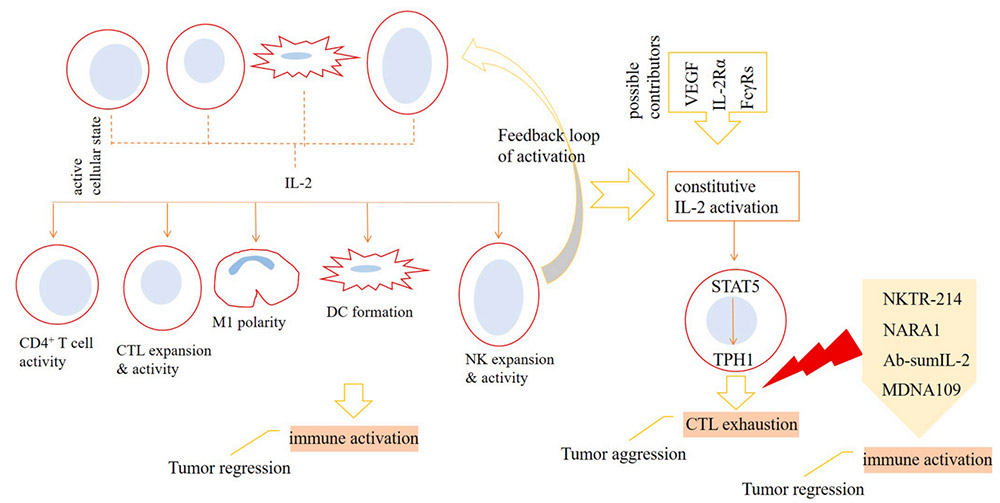
Inhibition of IL-2 on T cell effector function
To be specific, natural killer cells (NK), dendritic cells (DC), CD4+ T cells and CD8+ T cells upon activation release IL-2 into the tumor microenvironment (TME), which acts for strengthening immune activation against cancer. Polarization of macrophages toward anti-tumor type-1 phenotype (M1 cells), formation of DCs, and higher expansion and activity of cytotoxic T lymphocytes (CTLs) and NK cells are the consequences of IL-2 activity. The activation of IL-2 can cause the signal transducer and activator of STAT5 signal to be overactive, which further leads to the increase of TPH1 activity, and ultimately promotes the depletion state of CTLs. The activity of vascular endothelial growth factor (VEGF), IL-2Rα and melanoma-associated antigen 3 (MAGE-A3) may affect the activity of IL-2. To avoid this, the preferred strategy is to use IL-2 mutants that selectively act on IL-2Rγ, especially IL-2Rβ. Drugs currently undergoing clinical evaluation include NKTR-214, NARA1, Ab-sumIL-2 and MDNA109. Treatment with high-dose IL-2 can increase toxicity and interfere with the activity of endothelial cells (ECs) and effector T cells in the tumor microenvironment. This means that strategies related to cytokine therapy need to be adjusted, such as suppressing signals that interfere with the activity of the cytokine or using engineered IL-2 variants.
Treg cells can regulate autoreactive T cells and other immune cells. Once Treg cells cannot properly control the immune response, it will inevitably lead to autoimmunity and organ damage. Treg cells control the inflammatory response and become less efficient in inflamed tissues. Treg cells may even become unstable due to loss of FOXP3 expression and transform into a phenotype that is more characteristic of traditional CD4+ T cells. which is called "pre-T cell". In the early 1990s, IL-2 or IL-2R knockout mice showed severe autoimmunity rather than the expected immunodeficiency. In 2006, researchers concluded that Treg cells express high-affinity IL-2 receptors. The use of low-dose IL-2 in human autoimmune diseases may be a means to preferentially stimulate Treg cell activity without activating effector T cells.
In fact, it has now been determined that at a dose of 300,000-3,000,000 IU daily in the human body, IL-2 mainly stimulates Treg cells and is well tolerated. Since the lack of Treg cells is the core of the pathogenesis of most autoimmune diseases, low-dose IL-2 may have huge therapeutic potential and wide clinical applicability.
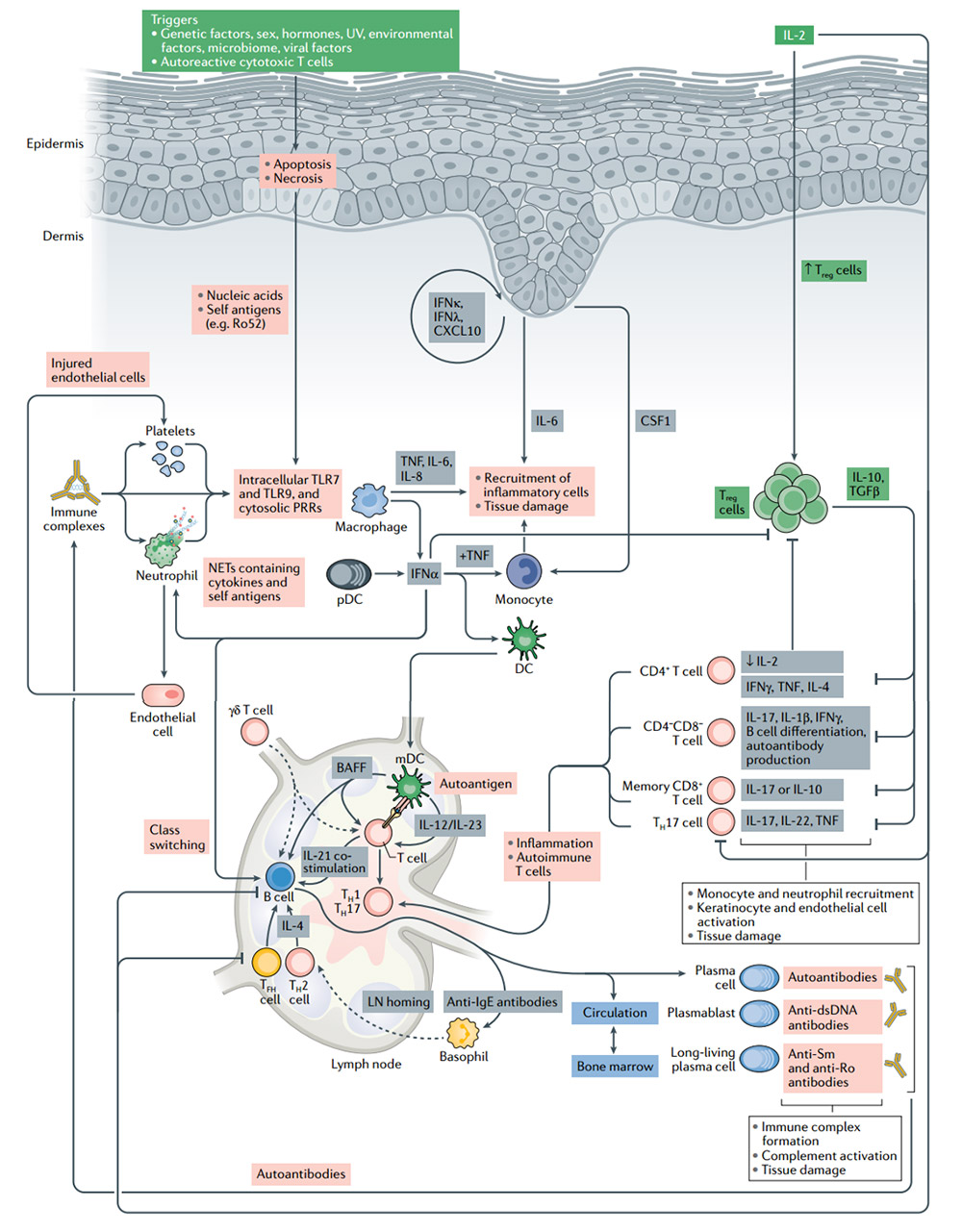
The pathogenesis of systemic lupus erythematosus (SLE) and the significance of IL-2 treatment
Treg cells have the inherent ability to induce peripheral tolerance and tissue regeneration. They are the cells of choice for cell therapy for patients with autoimmune diseases. In SLE patients, the number of Treg cells is reduced or their function is impaired. Low-dose IL-2 treatment leads to the expansion of Treg cells and directly inhibits B cells and TH17 cells, which leads to the control of autoimmunity.Without activating traditional T cells, the use of low-dose IL-2 can effectively expand and activate Treg cells, with good safety and clinical efficacy results are encouraging. However, Treg cells cannot survive severe inflammation. In these cases, IL-2 may have limited or no effect. Combination with drugs that target inflammatory pathways implicated in autoimmune disease pathogenesis and novel IL-2 therapies are necessary.
The translation of basic findings concerning IL-2 had a profound impact on the development of cancer immunotherapy. The administration of IL-2 as well as the adoptive transfer of antitumor T cells grown in IL-2 represented the first effective immunotherapies for cancer in humans and have provided one of the first curative systemic therapies for any solid tumor. The previous research on IL-2 has shown progress in the treatment of tumors and autoimmune diseases. It is necessary for Treg cell differentiation, immunosuppressive function, homeostasis, and survival. The new discovery of the biological activity of IL-2 has prompted the development of IL-2 curative effects, indications, and new formulations. At present, compared with the traditional IL-2 therapy based on dose selection, designing IL-2 variants with higher half-life, lower toxicity and higher efficacy is an important prospect.
ACROBiosystems has developed a series of IL-2 related proteins: IL-2, IL-2Rα (CD25), IL-2Rβ (CD122), IL-2Rγ (CD132), IL-2 R βγ heterodimer, IL-2Rαβγ heterotrimer with high purity, high stability and high bioactivity, which can be used to research the interaction between IL-2 and IL-2 receptors, immunization, antibody screening, etc., to facilitate the development of IL-2 related drugs.
>>> Click here to view IgG Fc products and verification data
>>> Click here to learn about IL-2 and IL-2 receptor proteins and verification data
1.Kolios, A.G.A., Tsokos, G.C. Klatzmann, D.Interleukin-2 and regulatory T cells in rheumatic diseases. Nat RevRheumatol (2021). https://doi-org-443--bjmu.99rl.xyz/10.1038/s41584-021-00707-x
2.Jamal Majidpoor, KeywanMortezaee. Interleukin-2 therapy of cancer-clinical perspectives. International Immunopharmacology (2021). https://doi.org/10.1016/j.intimp.2021.107836
3.Byungsuk Kwon. The two faces of IL-2: a key driver of CD8+ T-cell exhaustion. Cellular Molecular Immunology (2021). https://doi.org/10.1038/s41423-021-00712-w
4.Malek, T., Bayer, A. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 4, 665–674 (2004). https://doi.org/10.1038/nri1435
This web search service is supported by Google Inc.
