Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > [Inspiring Target] VEGF, the Everlasting Target for Antitumor Therapy 
More than a century ago, pathologists first observed several cases of an abnormal increase in vascularization of human tumors. Regarding this phenomenon, Ide et al. was the first to hypothesize the presence of vascular growth-stimulating factors produced from tumor cells to assist oncogenesis. In 1971, Dr. Judah Folkman proposed the novel idea of inhibiting tumor angiogenesis as a potential approach to treating cancers and other malignancies. However, it was only until 1983 that the relevant tumor angiogenesis factor, vascular endothelial growth factor (VEGF), was discovered and isolated as a vascular permeability factor that promoted angiogenesis. VEGF is an important regulator of angiogenesis in embryonic development, skeletal growth, and reproductive function and has been implicated in pathological angiogenesis associated with tumors, intraocular neovascular diseases, and other diseases. Despite its discovery in 1983, VEGF still ranks among the top five after Her-2, EGFR, CD3E and CD19 according to the Pharma Projects database. Over the past two decades, the critical role of VEGF in the regulation of physiological and pathological angiogenesis has been elucidated, resulting in VEGF becoming an ‘inspiring’ target for both current and innovative drugs.
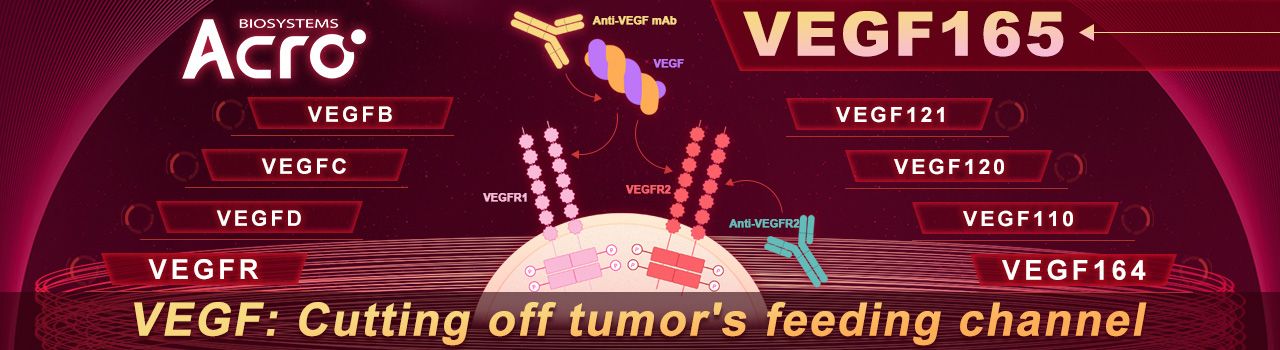
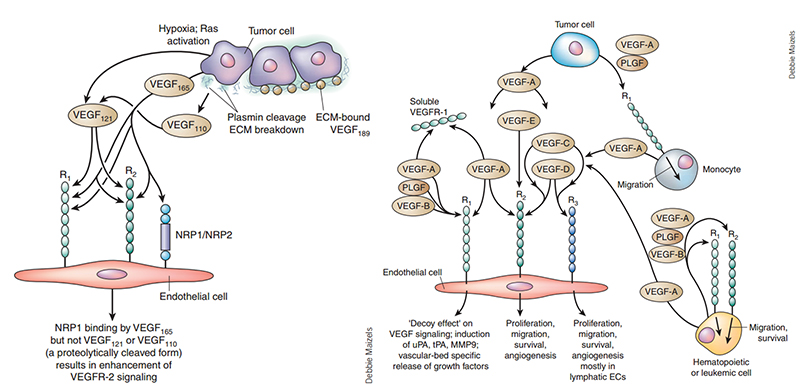
To fully appreciate the implications and potential of VEGF as a drug target, understanding the various roles of VEGF in physiologic processes is critical. The VEGF signaling system is complex, covering five major subtypes: VEGF-A, VEGF-B, VEGF-C, VEGF-D, placental growth factor (PIGF) with three tyrosine kinase receptors, VEGFR-1, VEGFR-2, and VEGFR-3. All subtypes share varying degrees of homology with VEGF, whereas the original VEGF specifically refers to VEGF-A, which plays an irreplaceable role in angiogenesis and maintenance. VEGF-A can be further separated into different isoforms: VEGF121, VEGF165, VEGF189, VEGF206. Each isoform has molecular properties, where VEGF165 was identified as the most abundant isoform with excellent bioavailability and biopotency properties.
Interaction of VEGF with receptors
Regarding VEGF receptors, there are also differences between VEGF receptors and its affinity and selectivity to different ligands. Among all receptors, VEGFR-2 plays the predominant role, mediating nearly all known cellular responses to VEGF, and is a major mediator of endothelial cell mitosis and survival, as well as angiogenesis and microvascular permeability.
Angiogenesis and microvascular permeability are physiological processes that influence the formation of blood vessels from pre-existing vessels. VEGF and its receptors mediate these processes that are critical in embryonic development and wound healing in adults. However, this signaling pathway is manipulated to facilitate tumor growth. During oncogenesis, VEGF is upregulated by oncogene expression, multiple growth factors, and hypoxia. Along with other growth factors produced by the tumor, VEGF causes an “angiogenic switch” to be turned on resulting in new blood vessel formation around the tumor and enable exponential growth. Due to the abnormal increase in angiogenesis, the resulting blood vessels formed are irregular in shape, curved and do not form venules, arterioles, and capillaries. Furthermore, these vessels leak and bleed, causing high interstitial pressure around the tumor area. The poor performances of these abnormal vessels result in poor blood flow, exacerbating hypoxia that results in further VEGF production and subsequently, angiogenesis.
The central role that VEGF plays in tumor angiogenesis makes it an effective target for antitumor therapy. Currently, an increasing number of companies are focusing on VEGF-targeted antibody drugs including monoclonal and bispecific antibodies(bsAb). Three representative drugs targeting VEGF are highlighted below.
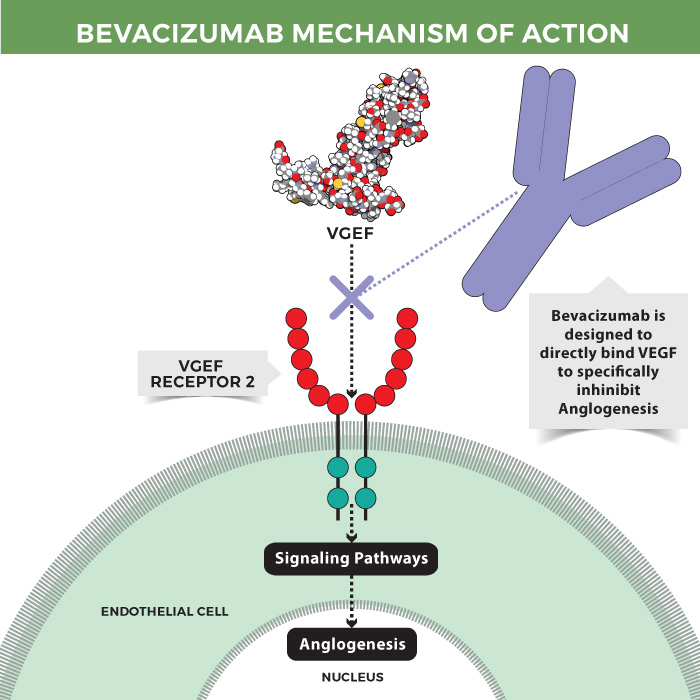
Mechanism of action of bevacizumab
Bevacizumab is a humanized anti-VEGF IgG1 monoclonal antibody. Developed by Roche, this drug is the world’s first anti-angiogenic drug that is indicated for use in a variety of tumors. The antibody directly binds to VEGF, thus inhibiting tumor-initiated angiogenesis and in turn, prevents tumor growth and metastasis.
Bevacizumab was approved by the FDA in 2004, approved in Europe in 2005, and launched in China in 2010 for the treatment of metastatic colorectal cancer. In 2015, the drug was approved for the treatment of non-small cell lung cancer in 2015. Currently, the approved indications of use encompass colorectal cancer, non-small cell lung cancer, glioblastoma, renal cell cancer, cervical cancer, ovarian cancer, fallopian tube cancer, peritoneal cancer, and many others.
One notable drug developed by Akeso is their PD-1/VEGF bsAb (AK112) that is based on the company’s unique TETRABODY technology. Currently, this drug has just entered the clinical stage as a combination therapy of PD-1 antibody and VEGF blocker for use in various tumor types including rencal cell carcinoma, non-small cell lung cancer, and hepatocellular carcinoma. Since both targets are blocked simultaneously with AK112, this drug can operate more effectively than a single agent resulting in enhanced antitumor activity.
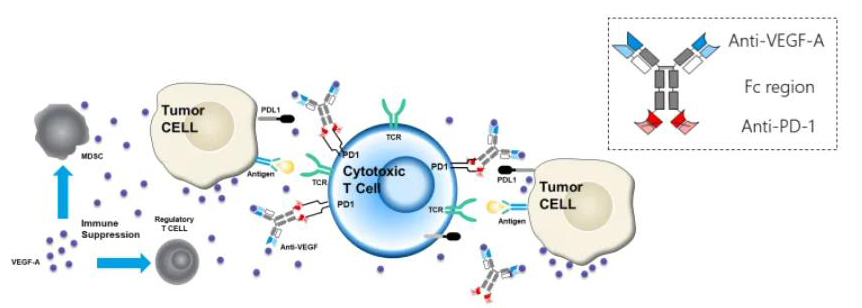
Mechanism of action of AK112
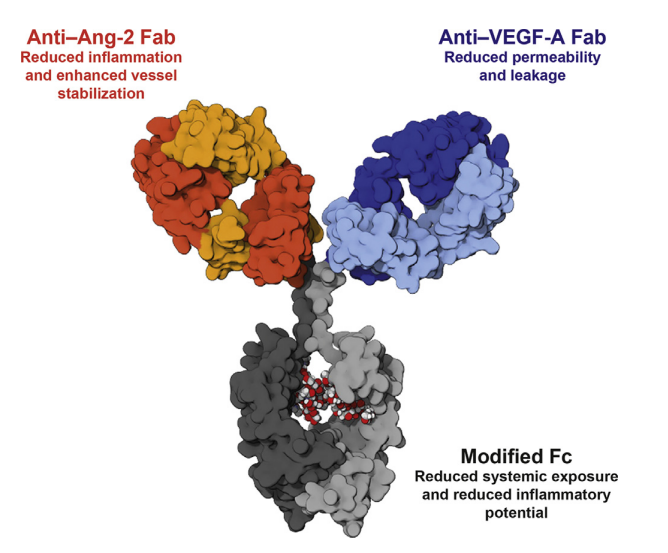
Structure of Faricimab
Roche also has a bsAB drug called Faricimab that targets Ang-2 and VEGF-A for use in ophthalmic diseases. Currently, not much information is known; however, Roche has stated that this drug has the potential to treat neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME).
In conclusion, VEGF has been considered as an underlying facilitator for tumorigenesis and disease progression in a broad range of human tumors. A therapy strategy targeting VEGF/VEGFR provides a unique entry point into the tumor vascular microenvironment. As such, VEGF has many properties that makes it an enticing target for potential therapeutics. The targeted therapy strategy targeting VEGF/VEGFR takes the tumor vascular microenvironment as the entry point. On one hand, it can cut off the nutritional source of tumor cells and inhibit tumor growth; On the other hand, the permeability of tumor drugs can improve the anti-tumor effect.
ACROBiosystems has launched a series HEK293-expressed VEGF family proteins with high-purity, multi-species, multi-tags, and highly bioactive VEGF family proteins that have been verified by ELISA/BLI/SPR/cell experiments, to help the development of VEGF/VEGFR antibody drugs.
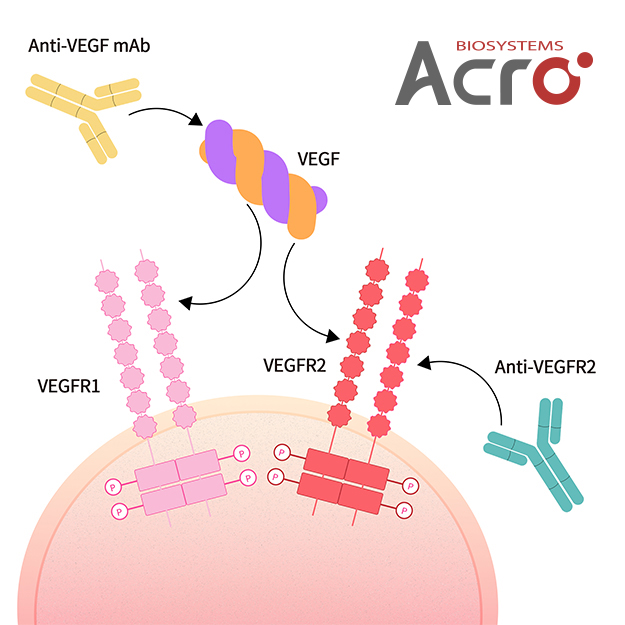
(Note: native VEGF is a 45 kDa heparin-binding glycoprotein with a homodimer structure, the correct and homogeneous structure is important to reduce the risk of drug development)
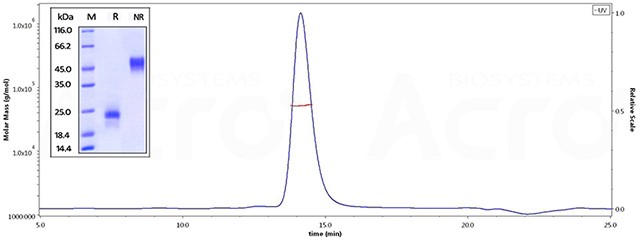
ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 98%. The purity of ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) is more than 95% in HP-SEC, and the molecular weight of this protein is around 40-55 kDa verified by SEC-MALS.
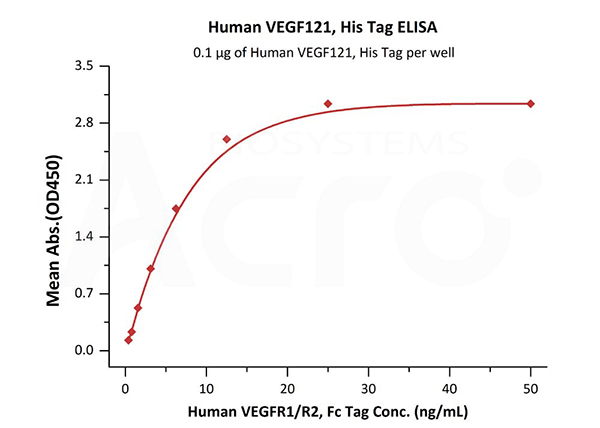
Immobilized Human VEGF121, His Tag (Cat. No. VE1-H5246) at 1 μg/mL (100 μL/well) can bind Human VEGFR1/R2, Fc Tag with a linear range of 0.4-6 ng/mL (QC tested).
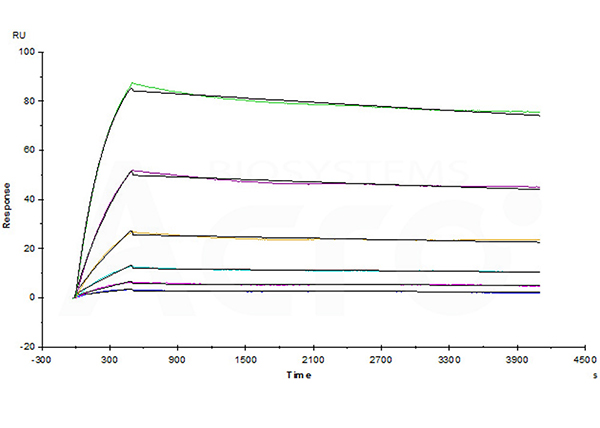
Avastin (Bevacizumab) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human VEGF165 Protein, His Tag (Cat. No. VE5-H5248) with an affinity constant of 1.03 nM as determined in a SPR assay (Biacore T200).
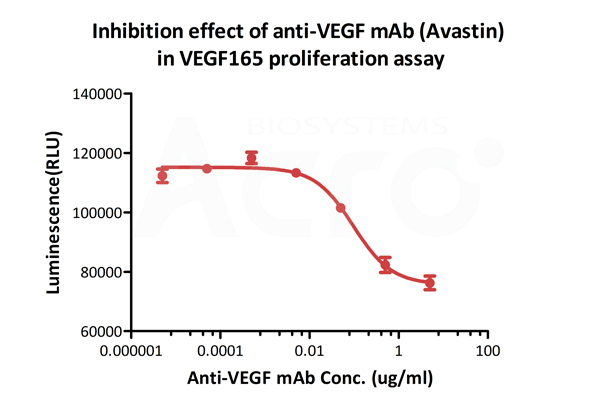
Inhibition assay shows that the proliferation effect of ActiveMax® Human VEGF165, Tag Free (MALS verified) (Cat. No. VE5-H4210) is inhibited by increasing concentration of anti-VEGF mAb (Avastin). The concentration of VEGF165 used is 20 ng/mL. The ED50 is 0.065-0.229 μg/Ml.
1. Napoleone Ferrara, Hans-Peter Gerber, et al. The biology of VEGF and its receptors. NATURE MEDICINE. 2003, 9(6): 669-676.
2. Peter Carmeliet. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005;69 (suppl 3):4–10.
3. Jayashree Sahni, Sunil S. Patel, et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema. American Academy of Ophthalmology. 2019: 1155-1170.
This web search service is supported by Google Inc.