
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Augmenting Immunotherapy Research: in Vitro Functional Assays using Primary Cells 
Immune checkpoint (CKP) drugs, as the forefront of immunotherapies, have become a hotspot in immune therapy due to their excellent clinical efficacy. The immense anti-cancer potential has firmly established itself as the forefront in pharmaceutical pipelines across the globe. In this highly competitive field, a comprehensive evaluation of promising candidates is required to maximize the chance towards obtaining FDA drug approval. In the research and development phase, a main primary indicator for candidate drug selection is therapeutic efficacy. However, directly performing in vivo studies could easily prove therapeutic efficacy and has a better correlation to subsequent clinical studies. This brings forth the question: why are in vitro studies necessary and which ones should be performed?
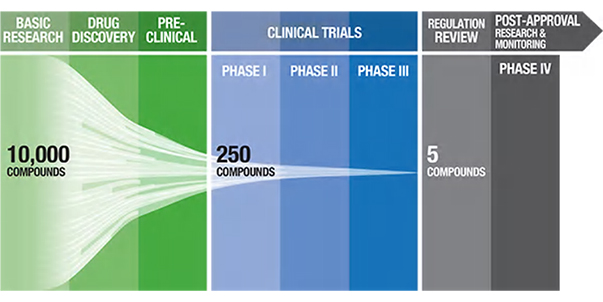
Figure 1. Pharmaceutical pipeline highlighting the number of potential candidate drugs to regulatory approval. 1
A key purpose for in vitro studies is in their ability to provide early data support, especially when performed with primary cells. Although this might seem redundant to in vivo studies, there is an important distinction. In vitro studies have experimentally controlled conditions, which provides irrefutable evidence supporting the proposed mechanism of action (MoA) and therapeutic efficacy. For CKP drugs, these assays are focused on several MoA evaluations: T-cell activation and expansion, cell-mediated activation, antibody-dependent cell-mediated cytotoxicity (ADCC), and others.
Within this article, several in vitro assays elucidating MoAs are explored, referring to specific cases.
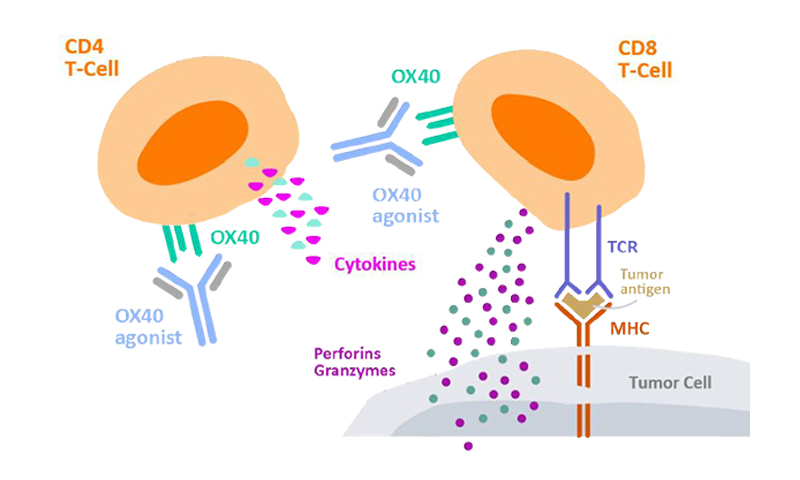
Figure 2. Cytokine secretion by CD4+ T cells to measure cell activation.2
Tcell activation and proliferation is a key MoA in CKP agonist drugs that rely on the promotion of adaptive immune response to coordinate an overall immune response against cancer cells. As an example, Kuang et al. performed several in vitro assays on their anti-OX40 antibody (IBI101) that targets colorectal adenocarcinoma.3 T-cell activation was measured through the quantitation of cytokine secretion. A 96-well plate coated with both anti-CD3 and IBI101 was prepared, with CD4+ T-cells added into each well along with cell media and anti-CD28 antibody. After incubation for 3 days, IL-2 secretion was quantified through ELISA. For T-cell proliferation, Far Red dilution was used for determination. PMBCs dyed through Far Red fluorescent reactive dye were placed into anti-CD3 coated well plates containing anti-CD28. Cells were incubated for 4 days with IBI101 and human IgG antibodies and stained with anti-human CD4/CD8 before analysis by flow cytometry.
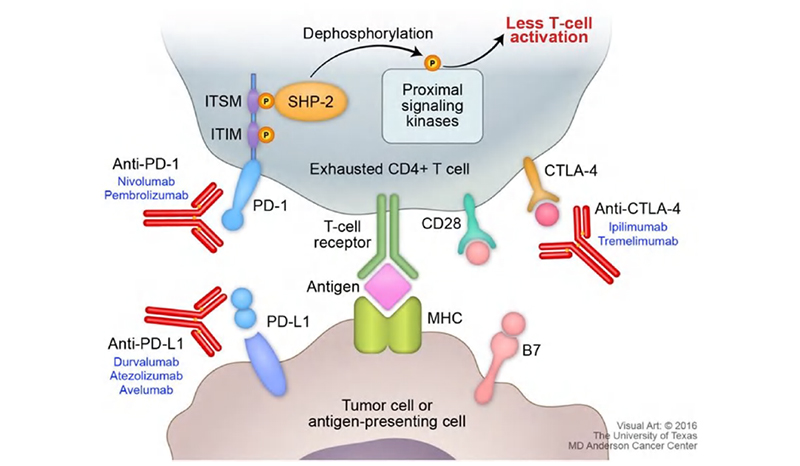
Figure 3. Figure on PD-1 pathway with cytokine secretion. 4
CKP inhibitors, contrary to agonists, work by blocking a specific pathway that mediates T-cell function, therefore requiring a more indirect method for testing called mixed lymphocyte reaction (MLR). This assay was performed by Wang et al. to characterize Nivolumab, an anti-PD-1 antibody that works as a CKP inhibitor.5 In this assay, dendritic cells (DCs) are co-cultured with T-cells. PD-L1 is abundantly expressed on the surface of DC cells which binds to PD-1 on T-cells to modulate T-cell activation. Through the addition of Nivolumab along with signaling cytokines such as IL-4 and GM-CSF, expansion of T cells is observed despite the presence of immune-modulating DCs. The resultant T cell expansion is quantified through cytokine secretion (IFN-γ) using a cytokine ELISA kit.
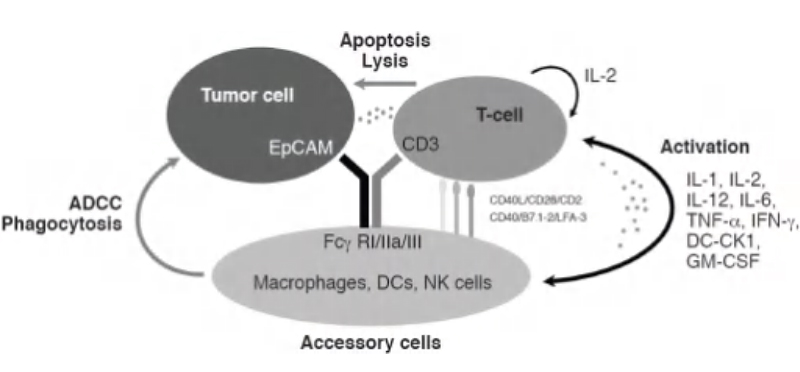
Figure 4. ADCC mechanism of action triggered by bispecific antibodies.5
The final MoA discussed in this article is antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is an inherent immune mechanism where a specific antibody binds to a target cell while engaging NK cells through its FCγR to initiate cell lysis. With the use of antibody drugs, this mechanism can be adopted for use in targeting certain cells.One such example is ATOR-1015, a bispecific antibody introduced by Kvarnhammar et al.6
ATOR-1015 targets both CTLA-4 and OX40, both of which are upregulated on activated T cells, in particular regulatory T cells (Tregs), that reside in the tumor microenvironment. With the use of antibody drugs, this mechanism can be adopted for use in targeting certain cells. One such example is ATOR-1015, a bispecific antibody introduced by Kvarnhammar et al.6 ATOR-1015 targets both CTLA-4 and OX40, both of which are upregulated on activated T cells, in particular regulatory T cells (Tregs), that reside in the tumor microenvironment. Treg depletion occurs through ADCC with ATOR-1015 binding to FCγRIIIa. To evaluate this MoA, an in vitro assay utilizing isolated NK cells with Tregs was performed. Tregs were first activated through anti-CD3/CD28 magnetic beads to express CTLA-4 and OX40. Afterwards, NK cells are cultured with the activated Tregs and ATOR-1015. As a result, ATOR-1015 binds to both NK cells and Tregs, inducing cell lysis of OX-40 and CTLA-4 expressing Tregs that is measured through lactate dehydrogenase content.
In vitro functional assays are a critical component in evaluating a candidate CKP antibody drug in the early stages of research and development. These assays provide scientific evidence in the verification of antibody activity, understanding of MoAs, and preliminary evidence supporting therapeutic efficacy, all of which are critical in decision-making in drug candidate selection. Although in vitro assays may differ significantly between types of drugs, ACROBiosystems offers an extensive catalog of related products to help develop in vitro assays, including immune checkpoint recombinant proteins, cytokines, quantitative cytokine ELISA kits, anti-CD3 antibodies, anti-CD28 antibodies, anti-CD3/CD28 antibody-coupled magnetic beads, and many others.
1. WEHI. Drug Discovery. 2022. [Online] Available at https://www.wehi.edu.au /research/research-technologies/drug-discovery.
2. Abbvie Oncology. 2021 [Online] Available at https://abbviescience.com/ oncology/biomarker-pathways/ox-40.
3. Kuang Z., Liu J., et al. Development and Characterization of a novel anti-OX40 antibody for potent immune activation. Cancer Immunol Immunother. 2020, 69(6):939-950.
4. Schvartsman G., Massarelli E., et al. Checkpoint inhibitors in lung cancer: Latest developments and clinical potential. Ther. Adv. in Med. Oncol. 8(6).
5. Wang C., Korman A., et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014. 2(9), 846-856.
6. Kvarnhammar A., Ellmark P., et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J Immunotherapy of Cancer. 2019, 7:103.
This web search service is supported by Google Inc.







