
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Facilitating Bispecific Antibody Drug Development through Quality Control Therapeutic antibody drug development is a long and exploratory process, with many risks and pitfalls. The use of high-quality and structurally defined recombinant proteins for screening and quality control can reduce the level of uncertainty throughout research and development. This is especially critical for new types of drugs, such as bispecific antibodies (bsAbs) which are regarded as the second generation of antibody cancer therapies. Back in 1960, the first bsAbs were developed through a thiol-redox method to couple Fab regions of two antibodies with different specificities. In 1975, Kohler and Milstein introduced hybridoma technology, setting off an antibody revolution. In the following few decades, antibody engineering including antibody fragment formats such as single-chain variable fragments (scFvs) and asymmetric antibody pairing approaches were introduced. Currently, the pharmaceutical pipeline for bsAb development has increased exponentially, with over 250 drugs entering preclinical trials and targeting various types of cancers. Alongside the different types of bsAbs engineering techniques, the mechanism of actions (MoA) has also evolved. To comprehensively test these new types of drugs, the selected quality controls to evaluate a final therapy must also take into consideration the MoA. As such, the tools used for quality control such as recombinant proteins must be of high-quality and consistent to fully evaluate the developed bsAbs.
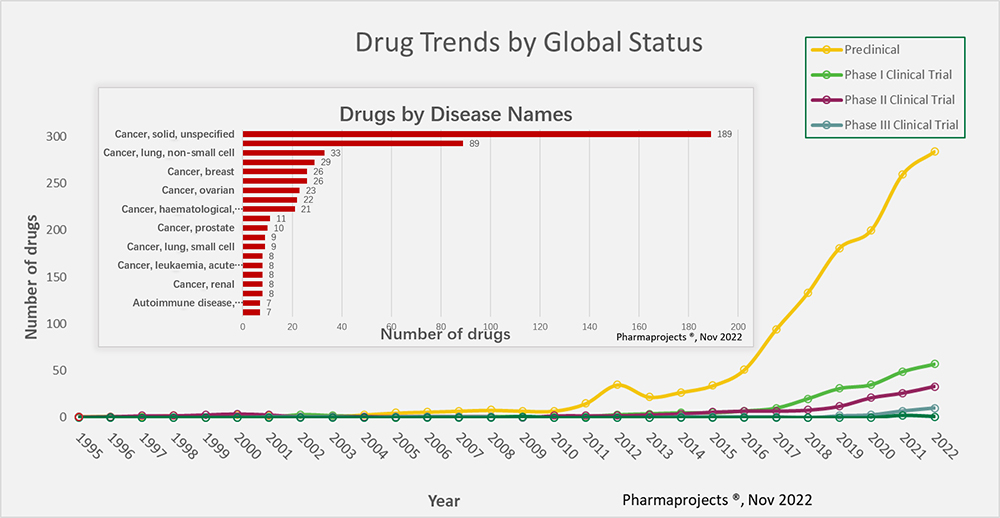
Figure 1. Trends from 1995 to 2022 for the development of bsAb drugs across the globe. (insert) bsAb target disease categories.
BsAbs have shown promise in its clinical effect due to its various drug MoAs. The most common MoA is as a cell engager, where the bsAb acts as a bridge between two or more cells to bring them into proximity. Other MoAs include ‘receptor inhibition/activation’ where the bsAb binds to multiple recepts on the same cell to allow for a higher affinity binding and increased effect. Co-factor mimetics are also bsAbs that bind to both co-factors to initiate the relevant signaling cascade. Lastly, ‘piggy backing’ is where one side of the bsAb targets a high-affinity receptor to help leverage a better position for the other bsAb side. This is helpful for bsAb drugs looking to cross the blood-brain barrier or to target low-affinity receptors that may not bind to monoclonal antibodies. Overall, with the dual specificity of bsAbs, the possible MoAs have been expanded greatly, furthering its potential as a cancer therapeutic.
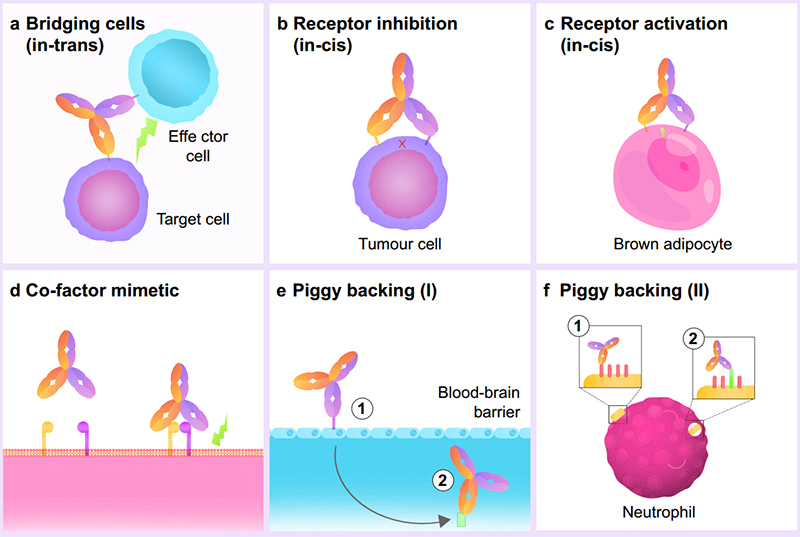
Figure 2. BsAbs fall into six subtypes based on their engineered biochemical activity
First, recombinant protein characterization and purity was performed to ensure appropriate conformation and quality. SDS-PAGE and SEC-MALS were performed on the CD3δ/CD3ε heterodimer, identifying more than 90% purity and the correctly formed heterogeneous complex. To evaluate bioactivity, SPR and ELISA studies were performed using human CD3 antibody (OKT3) and Human CD3E&CD3D heterodimer protein.
Based on the current approved bsAb drug market and pipeline, CD3-targeting bsAbs are a type of T cell engagers (MoA: bridging cells) and represent a fast-developing field in therapeutic antibody development. CD3 is a T cell co-receptor and protein complex that activates both cytotoxic T cell and T helper cells. By binding to both CD3 and a tumor-associated antigen, these bsAbs bridge T cells and tumor cells together to initiate tumor cell death, irrespective of T cell receptor specificity, co-stimulation, or peptide antigen presentation. Furthermore, the activation of T cells by CD3 bsAbs stimulates the secretion of a wide variety of cytokines to recruit immune helper cells to further enhance the anti-tumor cell effects.
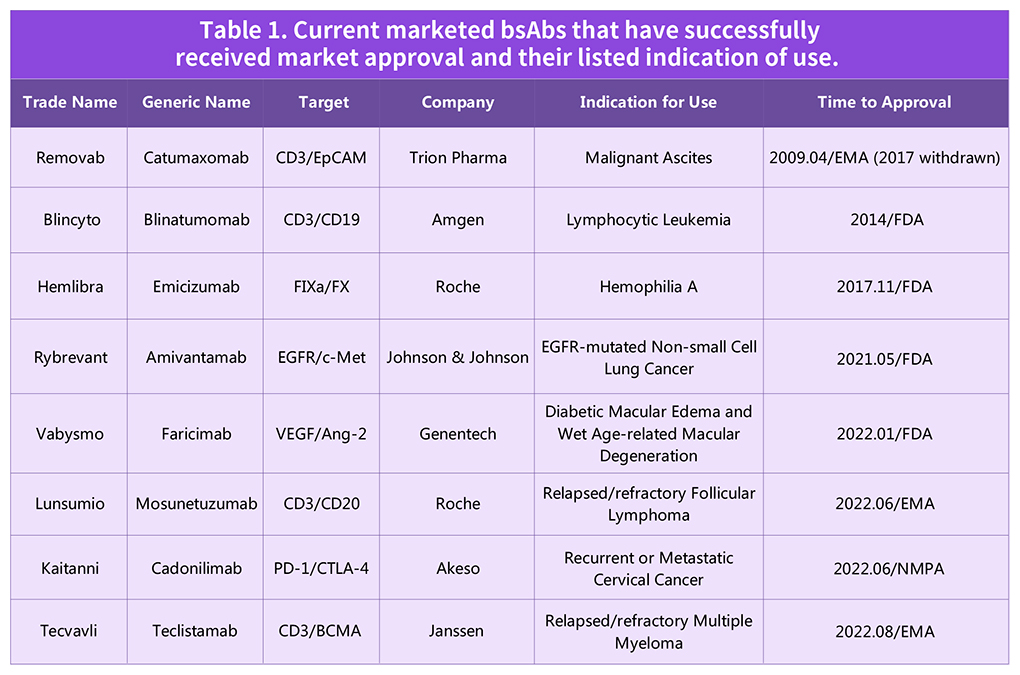
Table 1. Current marketed bsAbs that have successfully received market approval and their listed indication of use.
With a significant number of CD3-targeting bsAbs in the current immunotherapy pipeline, quality control using well-defined and high-quality CD3 proteins is critical. However, the recombinant expression of CD3 is relatively complex. There are 4 subtypes of CD3, including CD3δ, CD3ε, CD3γ, and CD3ζ. Several heterodimer pairs of CD3 form the necessary TCR-CD3 complex through the α/β chain of TCR. CD3-targeted bsAbs usually targets the CD3ε subunit in the heterodimer complex to activate the anti-tumor activity of T cells. However, in the process of recombinant expression, CD3ε and CD3δ can randomly form incorrect heterogeneous complexes. This makes it extremely problematic for the strict quality control necessary for CD3-targeting bsAbs.
To overcome the potential inconsistencies in CD3 protein expression, in-depth verification of the produced recombinant proteins must be performed. This includes protein structural characterizations, bioactivity studies and batch-to-batch analyses. Within these studies, a wide array of tests was performed to first characterize homogeneous CD3δ/CD3ε and CD3γ/CD3ε recombinant proteins including SDS-PAGE, SEC-MALS, SPR, and ELISA. Afterwards, a pharmacokinetic analysis of a T cell engager was evaluated using the CD3δ/CD3ε heterodimer.
Good binding affinities between CD3 recombinant protein and OKT3 was observed. Batch-to-batch analysis was also performed to ensure consistent bioactivity between CD3 heterodimer lots.
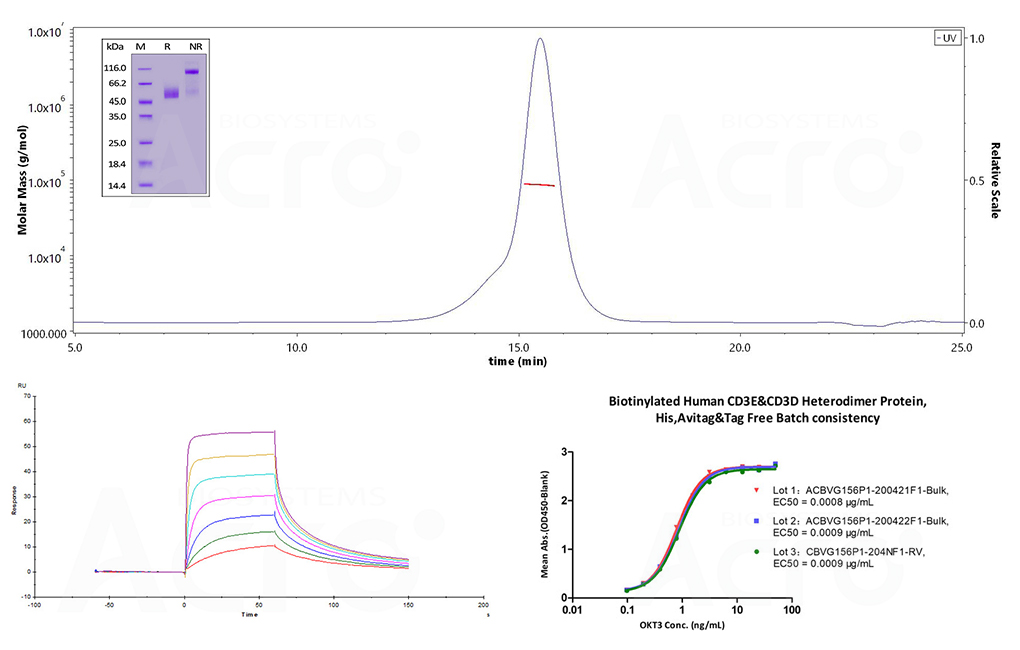
Figure 3. (Top) SDS-PAGE (insert) and SEC-MALS evaluation for structural homogeneity and purity. The purity of Human CD3E&CD3D Heterodimer Protein (Cat. No. CDD-H52Wa) is more than 90% using SDS-PAGE under reducing (R) and non-reducing(NR)conditions. The molecular weight is 80-90 kDa verified by SEC-MALS. (Bottom-left) SPR assay and (Bottom-right) ELISA were performed to evaluate the binding of Biotinylated Human CD3E&CD3D, His, Avitag & tag-free (Cat. No. CDD-H82W6) with anti-human CD3 antibody OKT3. The SPR assay identified an affinity constant of 22.5 nM with biotinylated human CD3 captured on a Biotin CAP-Series S sensor chip and evaluated on the Biacore T200. (Bottom-right) The linear range in the ELISA assay was identified to be 0.2 to 6 ng/mL. Lot differences were minimal, with EC50 differences between each lot less than 0.0001 ug/mL.
After confirming the quality of the protein, the same CD3 protein was used in the final ELISA study to evaluate the quality of a developed bsAb T cell engager (CD3 x BCMA). BCMA was first immobilized onto the well-plate while biotinylated CD3 recombinant protein was spiked into 10% human serum. Detection of HRP-conjugated streptavidin bound to the CD3 x BCMA antibody was identified to have a sensitivity of 15 ng/mL. As such, this study confirms that the bsAb T cell engager is bispecific to both BCMA and CD3.
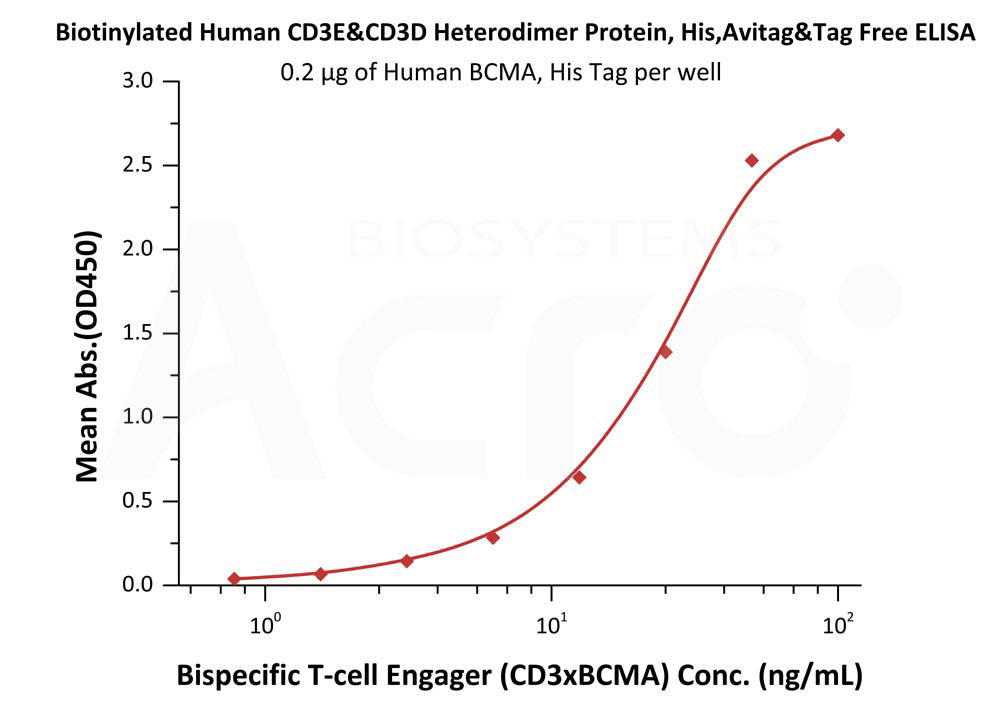
Figure 4. Immobilized Human BCMA, His Tag (Cat. No. BCA-H522y) was bound to a 96-well plate. Increasing concentrations of Bispecific T cell Engager (CD3 X BCMA) spiked in 10% human serum and Biotinylated Human CD3E&CD3D Heterodimer Protein, His,Avitag&Tag Free (Cat. No. CDD-H82W6) at 0.2 μg/mL was added to each well.
BsAbs are a relatively new type of drug in the field of targeted therapeutics. From the concept phase to clinical trials, many companies around the world are accelerating their research and development processes of these drugs, especially CD3-targeting bsAbs. This means that quality control of bsAb drugs becomes even more critical, especially as a novel immunotherapeutic. A key reagent for these quality control studies are recombinant proteins. To support bsAb research and its relevant quality control studies, ACROBiosystems strives to provide high-quality, bioactive, and consistent recombinant proteins including CD3 and many others.
References
1. Vafa O, T. ND, Perspective: Designing T-Cell Engagers With Better Therapeutic Windows. Frontiers in oncology (2020).
2. Labrijn AF, J. ML, R. JM, P. PWHI, Bispecific antibodies: a mechanistic review of the pipeline. Nature reviews. Drug discovery (2019).
3. Krishnamurthy A, J. A, Bispecific antibodies for cancer therapy: A review. Pharmacology & therapeutics (2018).
This web search service is supported by Google Inc.









