Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
>> Click here to learn more about DLL3&Notch proteins
Delta-like ligand-3 (DLL3) is a single-pass, type I transmembrane protein that plays a role in inhibiting the Notch signaling pathway. The Notch signaling pathway is a highly conserved intercellular signaling pathway that regulates embryonic development. Conventionally, the Notch signaling pathway is driven through the DSL family of Notch ligands. However, DLL3 is a divergent, otherwise termed ‘mutated’, member of the DSL family expressed due to a mutation in the Notch gene. This mutation drives the overexpression of DLL3, which in turn, inhibits the subsequent Notch signaling pathway within the DLL3-expressing cell and does not initiate the Notch pathway on adjacent cells.
| Spotlight Summary | |
 | |
| Protein Name | DLL3, Delta-like canonical Notch ligand 3 |
| Molecular Weight* | 50.4 kDa |
| AA Length | 619 |
| Accession # | Q9NYJ7-1 |
This dysregulation of the Notch pathway by DLL3 contributes to cell proliferation and reduced apoptosis, thus driving tumorigenesis. Below are a few key effects of DLL3 on the Notch signaling pathway:
1. DLL3 – Notch 1 Binding: This resulting binding leads to the inhibition of the Notch signaling pathway, downregulation HES1 and HEY1, both of which contribute to regulation of tumor cell.
2. DLL3 – Notch 2 Binding: Upregulation of expression levels of cell cycle proteins to increase cell cycle protein expression.
3. DLL3-Notch2-Notch4 Binding: Mediates cell proliferation and differentiation in ovarian cancer.
In general, DLL3 can bind to different Notch receptors and play multiple functions in cell proliferation, differentiation, and apoptosis. However, in different disease types, Notch receptor expression regulated by DLL3 can vary with the same resulting effect of driving tumor growth. As such, clarifying the interplay between DLL3 and Notch receptors in different tumorigenesis applications remains a key milestone in biological research.
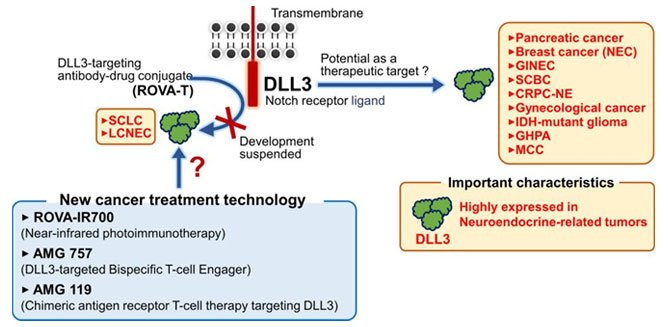
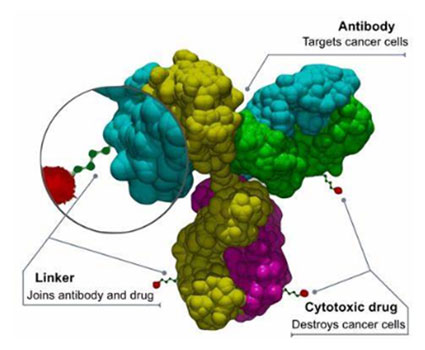
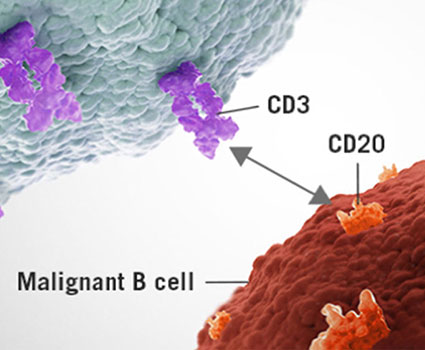
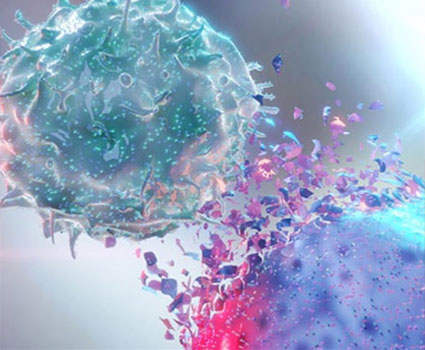
Figure 1. Potential Modalities to Target DLL3-expressing Cells
Despite the unclarity of DLL3’s biological function and role within Notch signaling, clinically, DLL3 is a clear biomarker for SCLC with its overexpression in over 75% in SCLC clinical trial populations. Furthermore, Notch mutations that drive DLL3 expression are exclusive to the lung, further emphasizing DLL3’s role as a biomarker for SCLC. As such, various therapies have been developed or are under development, including bispecific T-cell engagers (BiTEs), antibody-drug conjugates, and cell therapies, as seen in Table 1.
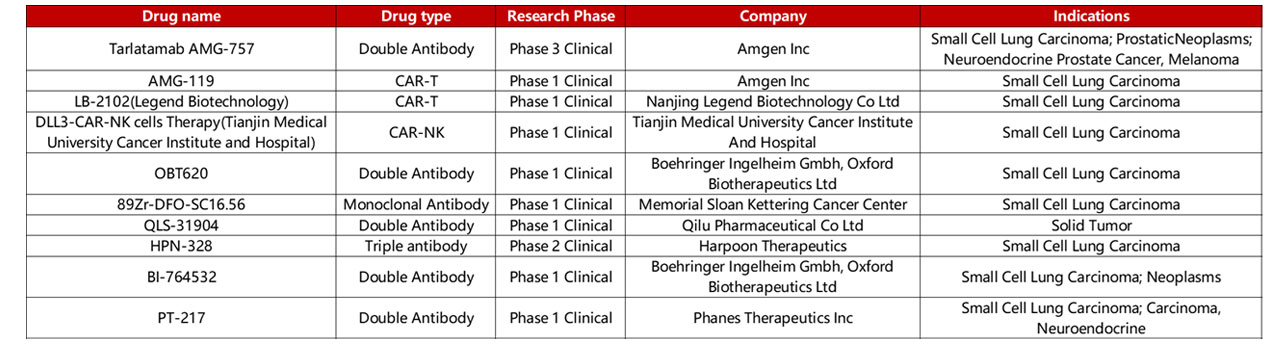
Table 1. DLL3 drug clinical information
Currently, the most promising DLL3 therapeutics are ADCs, BiTEs, and CAR cell therapies summarized in Figure 2. Each of these methods target DLL3, but rather than directly inhibiting DLL3 and its biological function, these therapeutics use DLL3 as a homing target to identify SCLC and other cancerous cells. Due to the specificity of DLL3 expression on SCLC cells, these therapies can accurately and precisely identify cancerous cells without affecting healthy, non-cancerous cells.
Antibody-Drug Conjugates (ADCs)
Antibody-drug conjugates are antibodies that carry a cytotoxic payload. By utilizing an anti-DLL3 antibody as the targeting method, delivery of cytotoxic payloads such as MMAE or DXD can be specifically delivered to DLL3-expressing tumor cells.
Bispecific T-cell Engagers (BiTEs)
Bispecific T cell engagers are a novel type of bispecific antibody that targets both DLL3 and CD3. By co-expressing CD3, the antibodies bind to both T cell and the DLL3-expressing tumor cells to initiate antibody-dependent cell cytotoxicity (ADCC).
Adoptive cell therapies are another targeted immunotherapy by modifying T cells to express an scFv complementary to DLL3. This enables T cells or other immune cells to seek out DLL3-expressing tumor cells and induce apoptosis through ADCC.
Clinically, the use of DLL3 is an up-and-coming biomarker that is finally entering the clinical stage. Further emphasis of these biologics should be focused more on manufacturing, finding methodologies to scale-up antibody production and optimize targeting, payload delivery, or improving ADCC interactions. Currently, there are numerous drugs from different companies that are entering the clinical stage, with a significant increase in DLL3-targeting drug pipelines, thus proving DLL3 is a significant direction for treatment of SCLC. Another aspect is cell therapy; beyond the conventionally approved FDA cell therapies targeting CD19 and BCMA, DLL3 could prove to be the first CAR target that enables cell therapy to combat solid tumors, presenting a significant milestone to cell therapies.
However, beyond its clinical use, biologically, that lack of understanding between DLL3 and Notch hinders its clinical application beyond SCLC. This is especially evident when differentiating between SCLC and non-SCLC, where seemingly opposing effects are evident between DLL3-expressing cells, yet still drive tumorigenesis.
To promote DLL3 and Notch signaling research, both in biologics manufacturing and in basic research, ACROBiosystems offers a wide array of DLL3 and Notch 1/2/3/4 protein products that are expressed using our HEK293 expression platform. Various labels are available, with validated applications including immunization, biopanning, and biofunctional verification assays.
1. Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139(23):4365–73.
2. Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17(11):722–35.
3. Steinbuck, Martin Peter, and Susan Winandy. "A review of notch processing with new insights into ligand-independent notch signaling in T-cells." Frontiers in immunology 9 (2018): 1230.
4. Smit MAD, Borghaei H, Owonikoko TK, et al. Phase 1 study of AMG 757, a half-life extended bispecific T cell engager (BiTE) antibody construct targeting DLL3, in patients with small cell lung cancer (SCLC). J Clin Oncol. 2019;37:TPS8577.
5. Frederickson RM. A new era of innovation for CAR T-cell therapy. Mol Ther. 2015;23:1795-1796.
This web search service is supported by Google Inc.