
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Solutions for Evaluation of CAR Expression
The chimeric antigen receptor T (CAR-T) cell therapy is a new treatment for a variety of cancers. The idea is to take the T-cells from the patient, and genetically engineer the cells to express a chimeric antigen receptor (CAR) which recognizes a specific tumor-associated antigen (TAAs). As a result, the CAR-expressing T cells, when reintroduced into the patient's body, will target and eliminate the TAA-expressing tumor cells.
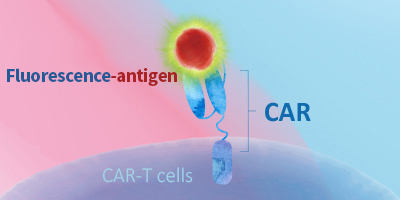
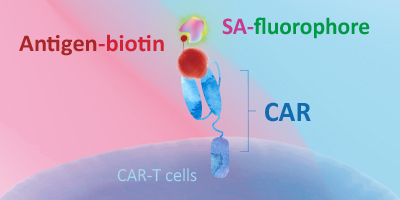
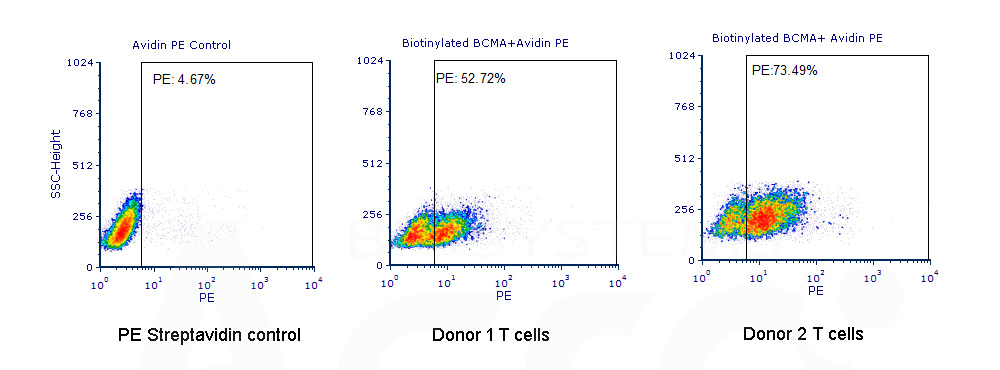
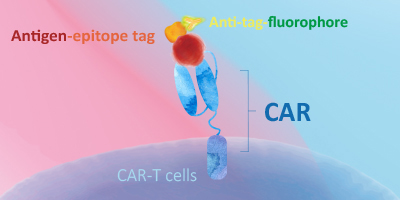
Evaluating CAR expression is an essential step in the production of CAR-T cells. This is often done by flow cytometry, using protein L, anti-Fab antibodies or target antigens as detection methods. Among these common choices, target antigens are widely considered to be the best option, because it offers high specificity and minimal background staining.
ACROBiosystems has developed an extensive collection of recombinant proteins to support CAR-T therapy development. This growing list of proteins includes many fluorescent-labeled target antigens and pre-biotinylated proteins that are uniquely suitable for evaluation of CAR expression. In addition, we also supply difficult-to-express proteins such as BCMA, CD19, ROR1, and EGFRVIII.


This web search service is supported by Google Inc.








