 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Special Topic on Deep Interpretation of GMP Product Quality--Topic 4

In the ever-evolving landscape of biopharmaceutical industry, cell and gene therapy (CGT) emerges as a highly compelling and rapidly advancing field of medicine. At the forefront of these breakthroughs includes autologous and allogeneic cell therapies, personalized treatments involving a patient's own cells or donor cells for a select population. Nevertheless, a challenge facing the CGT industry lies in ensuring a secure and reliable supply chain for critical Good Manufacturing Practice (GMP)-grade raw and ancillary materials for CGT manufacturing. This article explores critical aspects of maintaining a material supply chains, including supply chain management, warehouse inventory management, transportation logistics, and adherence to international trade compliance.
When considering GMP-grade raw materials for CGT manufacturing, it is critical to ask whether a supplier can stably supply the raw material needs throughout clinical trials and into commercialization. As the success of a CGT heavily relies on the quality and consistency of these ancillary materials, making the choice of a supplier a crucial decision.
One main point to consider when evaluating a supplier is their supply chain management for GMP-grade raw materials, including robust supply agreements, stringent supplier management practices, and transparent communication with suppliers.
First and foremost, robust supply agreements are essential to ensure the availability of high-quality GMP-grade ancillary materials. These agreements should outline quality standards, delivery schedules, and contingency plans to mitigate potential supply chain disruptions. For instance, securing large size batches reserved for one customer and validating a second supplier can further secure the supply chain and final product stability, especially when scaling up from clinical trials to large-scale commercial manufacturing.
Secondly, stringent supplier management practices are critical to guarantee the quality and consistency of GMP-grade ancillary materials. This involves thorough qualification collection, on-site audits, and the establishment of a GMP-grade quality management system to ensure compliance with international standards and regulatory guidelines.
Transparent communication with suppliers is another vital aspect of supply chain management for GMP-grade ancillary materials. Establishing a defined communications lead and issue escalation pathway for key suppliers can ensure that both parties are aligned on key priorities and potential challenges. Regular business review meetings with key suppliers can further enhance transparency and collaboration, ultimately strengthening the supply chain.
In the intricate landscape of GMP-grade ancillary materials for CGT manufacturing, the pivotal role of warehousing and inventory management cannot be overstated. These functions are crucial for ensuring the safe storage, precise distribution, and efficient turnover of materials and products, ultimately mitigating the risk of supply chain disruptions.
Given the hypersensitive and vulnerable nature of GMP-grade materials, warehouses must adhere to stringent audits. These audits encompass controls over critical factors such as temperatures, humidity, cleanliness, expiration, turnover rates, and timely shipment processes. The emphasis on precise inventory management with full lifecycle traceability becomes the foundation to guarantee the safety and quality of raw materials.
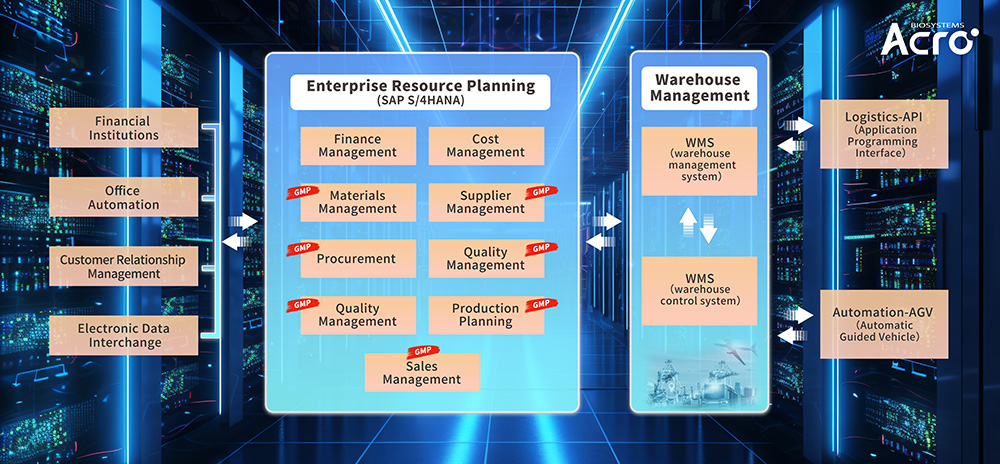
ACROBiosystems' Comprehensive Management System:
To streamline operations, we established an Order and Material Product Management System (OA-CRM-ERP-WMS-WCS). This system includes features such as business opportunity management, automated order conversion audits, unique identification for material products, GMP product classification, and end-to-end product lifecycle tracking. By leveraging barcodes, QR codes, and RFID labels, we ensure complete lifecycle traceability and management to guarantee our customers a consistent and stable supply of high-quality GMP raw materials.
Global Warehousing Presence
To maintain delivery of our products to customers globally, establishing warehouses and logistic centers are critical. We maintain three different centers in the United States, EU and China, ensuring product delivery to our customers as fast as possible.
• North American International Logistics Center: Operational for over 10 years on the East Coast, this center is ISO9001 and ISO13485 certified, with a finished product warehouse capacity exceeding 500,000 units.
• European Logistics Center: Serving over 90% of European customers, this center aims for ISO9001 and ISO13485 certifications by 2024, enhancing the speed of services.
• Beijing Logistics Center: Operational for over 13 years and holds ISO9001 and ISO13485 certifications. This center in China oversees global warehousing logistics, implementing automated low-temperature storage management with a capacity of up to 1.5 million units.
Robust Inventory Management
Regular inventory checks and quality inspections, aligned with internal control and audit standards, are integral to our approach. The inventory management system incorporates safety stock alerts and an AI-driven Material Requirements Planning (MRP) mechanism for timely procurement, ensuring a consistent supply in the face of unforeseen circumstances and market fluctuations.
Optimizing Warehousing Efficiency
We continually optimize warehousing layout design based on customer needs and future plans. Digitalization, automation, and intelligent transformation enhance efficiency and space utilization. Adhering to GMP requirements, storage areas are strategically divided, and materials are classified to prevent cross-contamination, ensuring a secure and high-quality storage environment.
International transportation logistics serve as the crucial link connecting various facets within the raw material supply chain. Given the sensitivity of GMP-grade raw materials, environmental factors such as temperature, humidity, and vibrations during transportation can exert irreversible impacts on their quality, thereby escalating logistics costs and complexity. Therefore, establishing a reliable transportation logistics system is of utmost importance. A stable logistics system ensures the timely and secure delivery of GMP-grade raw materials, enhancing the efficiency and reliability of the CGT manufacturing supply chain.
In light of this, we have strategically partnered with globally renowned and highly reliable logistics suppliers. These logistics partners bring extensive experience in the biopharmaceutical industry and possess a specialized understanding nature of GMP-grade raw material. They offer secure and reliable transportation services across different temperature ranges, ensuring compliance with industry regulations and traceability, aligning with the transportation specifications for critical GMP materials. To our Americas and Europe customers, more than 90% of deliveries are accomplished within three days. Globally, 97% of shipments are in transit within three working days.
Furthermore, a temperature monitoring system is implemented throughout the transportation process, enabling continuous monitoring and tracking. This system detects anomalies promptly, allowing for immediate actions to ensure temperature control remains within acceptable ranges, safeguarding the safety and integrity of the raw materials during transportation.
In addition, we have a thoroughly developed plan encompassing transportation and route planning, ensuring the timely delivery of GMP grade raw materials for customers. Tailoring packaging materials and containers based on the unique characteristics of different materials and destination requirements, the company selects the optimal transportation mode, including air and land transportation. Transportation times are thoughtfully arranged to minimize both the time and risk associated with raw material transportation.
Managing the international movement of critical raw materials is a crucial aspect of supply chain management in the global therapeutic technology landscape of CGT. Yet, navigating international trade remains challenging due to diverse regulatory landscapes and trade policies across countries and regions. Distinct regulations and tax policies for CGT products vary, potentially restricting certain imports or exports. This introduces extra compliance costs and time delays for biopharma. A strategic approach to evaluating and managing international import and export operations is essential to minimize risks, enhance efficiency, and ensure a stable supply of key GMP-grade raw materials. A fundamental understanding of the import-export regulations and tax policies in the target market is the key to successful international trade.
Table 1: List of Relevant International Regulations
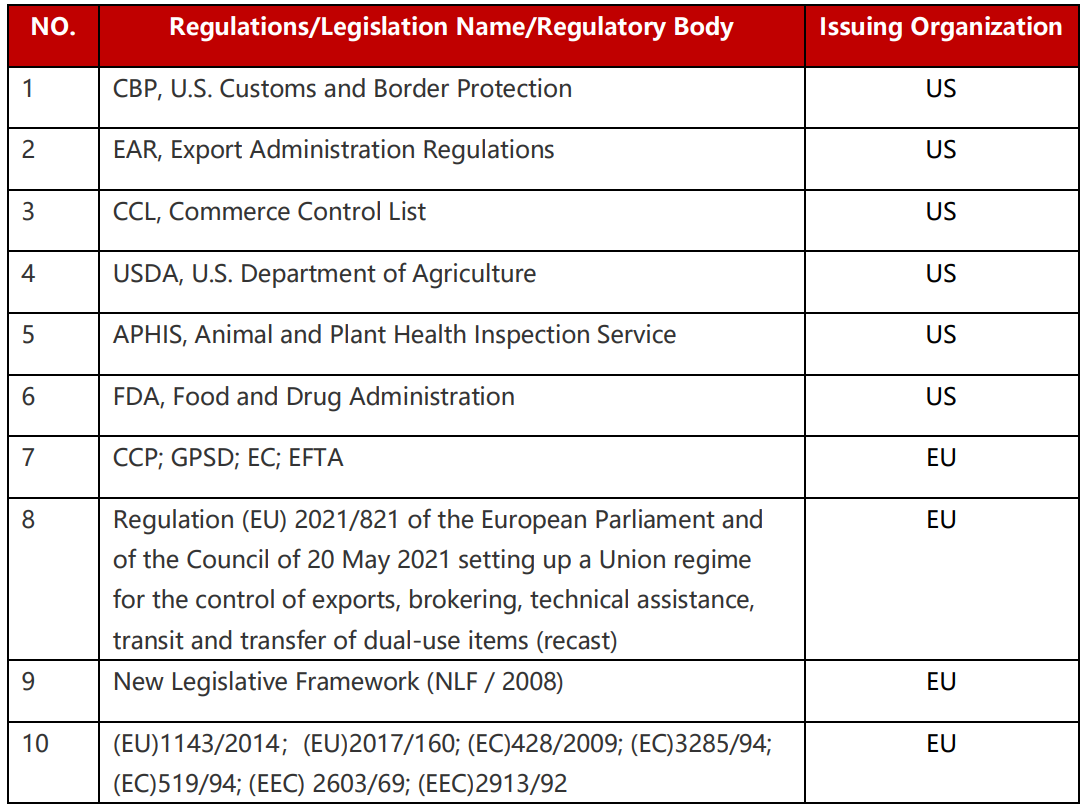
We are unwavering in our commitment to continuous learning, understanding, and adherence to pertinent international trade laws, regulations, and standards. Our emphasis is on ensuring the compliance of its import and export operations. This involves familiarizing itself with the import-export specifications and procedures of the targeted market, establishing robust document management, and filing systems to minimize potential legal risks.
Internally, we have nurtured stable partnerships within its supply chain network, encompassing suppliers, agents, and service providers. These enduring and positive collaborations guarantee the stability and reliability of the supply chain. Regular assessments of existing service providers are conducted to ensure their capability to consistently deliver high-quality services. In addition, when required, new service providers are carefully evaluated based on their qualifications, reputation, and professional competence, particularly in handling unforeseen emergencies.
We also places significant emphasis on information sharing and adopting localized business practices. By maintaining close ties with relevant departments, partners, and local governments, ACROBiosystems advocates for specialized, localized services. This proactive approach enables them to stay abreast of changes and trends in international trade policies promptly, facilitating timely adjustments and informed decision-making in their business operations. The overarching goal is not only to navigate the complexities of international trade but to do so in strict compliance with internal laws and regulations, ensuring a seamless and lawful global operation.
This article focuses on how ACROBiosystems addresses the increasing demand for high-quality materials in CGT manufacturing. It highlights key elements of their global supply chain system, emphasizing robust management of GMP grade products. The company employs stringent supplier agreements, transparent communication, and advanced warehousing through its Comprehensive Management System. This system includes features like end-to-end product tracking and efficient inventory management to meet the growing needs of CGT manufacturing. Our strategic approach extends to global warehousing, transportation partnerships, and a commitment to international trade compliance, positioning them as a proactive contributor to the evolving field of CGT.




This web search service is supported by Google Inc.
