Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
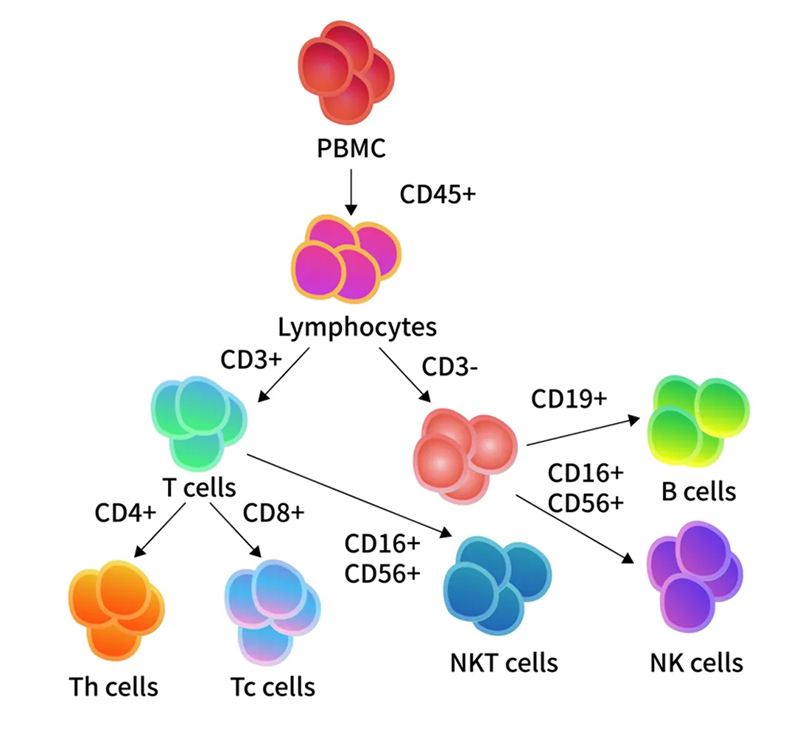
> Insights > Innovative Advances in Immune Cell Profiling: ClinMax™ Human TBNK 6-color Cocktail Flow Panel Peripheral Blood Mononuclear Cells (PBMCs) are critical immune cells isolated from peripheral blood. These cells are integral to the immune response, tasked with identifying and eliminating foreign pathogens and abnormal cells to maintain health. Within PBMCs, lymphocytes comprise approximately 70% to 90% of the total cell population. These lymphocytes are categorized into T cells (CD3+), B cells (CD3-CD19+), and NK cells (CD3-CD16+CD56+). T cells are further divided into helper T cells (CD3+CD4+) and cytotoxic T cells (CD3+CD8+) based on CD4 and CD8 expression.
PBMCs are crucial in both clinical and research settings. They serve as a primary cell source for cell therapies such as CAR-T and are essential in applications ranging from in vitro biomarker detection and tumor immunology to toxicology and rare disease research. Monitoring the proportions of various PBMC subpopulations is key in immune cell therapies, such as CAR-T cell therapy.
CAR-T cell therapy typically involves three critical stages: evaluating the cell source, preparing the cell product, and assessing therapeutic outcomes. As the primary source of CAR-T cells, understanding the proportion of different cell types and subpopulations within PBMCs is vital for guiding cell therapy processes, assessing cell purity, and evaluating in vivo efficacy. The proportion of different cells and subpopulations in PBMCs or final products is a crucial consideration for the safety and efficacy of CAR-T cell therapies. Regulatory agencies such as, the US Food and Drug Administration (FDA), the China National Medical Products Administration and the European Medicines Agency emphasize the need for detailed cellular phenotype analysis, including percentages of CD3+, CD4+, CD8+ T cells, and NK cells. Quality control standards for CAR-T cell products involve characterization, uniformity, and purity testing to ensure product consistency and quality.
Technologies for immune cell profiling include flow cytometry, next-generation sequencing, qPCR, and chip-based assays. Among these, flow cytometry is the preferred method due to its high throughput, efficiency, and precision. Flow cytometry identifies and sorts of cells based on surface markers through specific antibody binding and fluorochrome conjugation, enabling accurate quantification of immune cell subpopulations. This technique's high sensitivity and specificity make it widely used in basic research and clinical sample analysis.
ACROBiosystems is proud to present the ClinMax™ Human TBNK 6-color Cocktail Flow Panel (Cat. No. ICA-A001), a state-of-the-art solution for immune cell profiling. This panel, developed using rigorous methodological validation and proprietary technology, offers a rapid, reliable means of detecting T cells, B cells, and NK cells in human whole blood or PBMCs.

This web search service is supported by Google Inc.