 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Tecelra® Approval Marks Milestone in Solid Tumor Immunotherapy: Advancing TCR-T Cell Therapy The landscape of cancer immunotherapy has witnessed a significant breakthrough with the FDA's recent approval of Tecelra ® (Afamitrisgene autoleucel,Afami-cel), developed by Adaptimmune Therapeutics. This approval marks the first T-cell receptor (TCR) therapy for solid tumors, specifically targeting unresectable or metastatic synovial sarcoma in adults. Tecelra's success not only offers new hope for patients with limited treatment options but also paves the way for broader applications in solid tumor therapy, an area where progress has been notoriously challenging.
Tecelra represents a novel class of engineered autologous T-cell therapies that target the MAGE-A4 antigen, a cancer germline antigen expressed in many tumors but absent in healthy tissues. Unlike chimeric antigen receptor (CAR) T-cell therapies, which recognize surface antigens, TCR-T cell therapies like Tecelra can identify intracellular antigens presented by major histocompatibility complex (MHC) molecules. This fundamental difference expands the range of potential targets and offers new avenues for addressing solid tumors.
The distinction between CAR-T and TCR-T therapies lies not only in their target recognition but also in their antigen scope and MHC dependence. While CAR-T cells are engineered to recognize specific surface proteins, TCR-T cells can target a broader range of antigens, including those within the cell. However, this advantage comes with increased complexity, as TCR-T therapies require patients to possess specific HLA antigens and have tumors expressing the target antigen, in this case, MAGE-A4.
The FDA's approval of Tecelra was based on compelling results from a Phase 2 trial involving 44 patients with synovial sarcoma. The study demonstrated an overall response rate of 43%, with 17% of patients maintaining their response for at least 12 months. The median duration of response was 36 weeks, ranging from 9.1 to 164.9 weeks, and the median progression-free survival was 21.1 weeks. These outcomes are particularly significant given the limited treatment options and poor prognosis associated with synovial sarcoma, a rare soft tissue cancer affecting approximately 1,000 people annually in the United States.
While the efficacy data is promising, it's crucial to consider Tecelra's safety profile. Common side effects include cytokine release syndrome (CRS), pyrexia, decreased lymphocyte count, and fatigue. Serious adverse events occurred in 39% of patients, with CRS being the most frequent at 20%. The FDA approval includes a boxed warning for CRS, neurotoxicity, and hematologic toxicity, underscoring the need for careful patient monitoring and management of potential side effects.
Tecelra's success in synovial sarcoma opens new possibilities for applying TCR-T therapies to other solid tumors. The ability to target intracellular antigens, coupled with the potential for improved tumor penetration and enhanced persistence within solid tumors, positions TCR-T therapy as a promising approach for cancers that have long evaded effective immunotherapy.
Ongoing research is exploring additional tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) to expand the scope of TCR-T therapies across different cancer types. For instance, the NY-ESO-1 antigen has shown promise in clinical trials for synovial sarcoma and melanoma, illustrating the potential breadth of TCR-T applications.
The production of TCR-T therapies like Tecelra involves complex, patient-specific manufacturing processes that present unique challenges. Each batch must be customized for individual patients, requiring precise coordination and timing. Ensuring consistent potency and purity across batches while scaling production to meet demand presents significant logistical and quality control challenges. These factors contribute to the high production costs associated with personalized cell therapies, a hurdle that must be addressed for wider adoption and accessibility.
Despite these challenges, the field of TCR-T therapy continues to evolve rapidly.
A key area of future development in TCR-T therapy lies in the exploration and validation of new targets. These targets can be broadly categorized into two groups: Tumor-Associated Antigens (TAAs) and Tumor-Specific Antigens (TSAs).
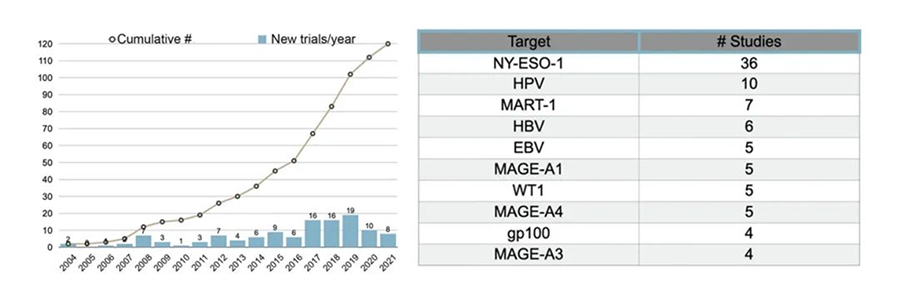
Figure 1. Trends in the number of TCR-T clinical trials and popular targets.
• Tumor-Associated Antigens (TAAs)
TAAs are overexpressed in tumor tissues but have limited expression in normal tissues. They can originate from tumor-derived tissues (tissue differentiation antigens) or germline tissues (cancer germline antigens). While TAAs are attractive therapeutic targets in TCR-T research, their expression in normal tissues poses a risk of on-target/off-tumor toxicity, necessitating careful selection and validation.
- Tissue Differentiation Antigens (TDAs): TDAs include melanoma antigens like MART-1 and glycoprotein 100 (gp100), as well as carcinoembryonic antigen (CEA). Several TCR-T therapies targeting these antigens are currently in clinical trials. A notable success in this category is Tebentafusp, a soluble affinity-enhanced TCR targeting gp100 fused to an anti-CD3 single-chain variable fragment. Tebentafusp has been approved for treating HLA-A*02:01-positive patients with metastatic uveal melanoma, with a Phase 3 randomized trial demonstrating significant overall survival benefit.
- Cancer Germline Antigens (CGAs): Most clinical trials targeting CGAs focus on members of the MAGE-A protein family and New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1). TCR-T cells targeting NY-ESO-1 have shown particularly promising results in clinical trials, especially in treating melanoma and synovial sarcoma. Across five trials involving 107 patients, the overall response rate (ORR) averaged 47% (ranging from 20% to 67%), with eight complete responses and 40 partial responses reported, all without significant adverse effects.
• Tumor-Specific Antigens (TSAs)
TSAs, also known as neoantigens, are unique to tumor cells and associated with tumorigenesis, either through mutations or viral induction. Targeting these neoantigens offers a lower toxicity risk due to their absence in normal tissues, making them highly attractive targets for future TCR-T therapies.
- Mutation-Associated Neoantigens: These neoantigens arise from non-synonymous mutations linked to oncogenic genetic instability. Of particular interest are "public" neoantigens, derived from frequently mutated driver genes like TP53, KRAS, or PIK3CA, which are shared by multiple patients with a specific HLA allele. Several ongoing clinical trials are evaluating TCR-T therapies targeting these public neoantigens, such as TCR-T cells targeting KRAS-G12D in pancreatic cancer and KRAS-G12V in other cancers.
- Viral Antigens: Some cancers are driven by viral infections, such as human papillomavirus (HPV), hepatitis B virus (HBV), Merkel cell polyomavirus (MCPyV), and Epstein-Barr virus (EBV). These virally induced cancers present unique opportunities for TCR-T therapy. For instance, a clinical trial using HPV16-E6-specific TCR-T cells showed no significant toxicity in 17% of patients (2 out of 12), further highlighting the potential of targeting viral antigens in cancer treatment.
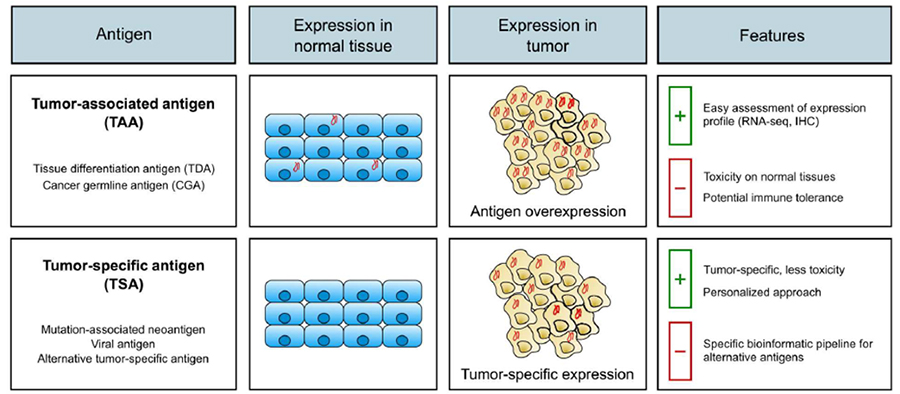
Figure 2. Types of Target Antigens in TCR-T Cell Therapy
The approval of Tecelra marks a significant milestone in cancer immunotherapy, offering new hope for patients with synovial sarcoma and potentially opening doors for treating a wide range of solid tumors. This breakthrough underscores the transformative potential of TCR-T cell therapies in oncology. As research progresses, we can expect further refinements in target identification, engineering strategies, and combination approaches. While challenges remain in manufacturing, scalability, and managing side effects, Tecelra's success may accelerate innovation in the field. The continued development of TCR-T therapies holds great promise for improving outcomes across a range of previously intractable cancers, potentially revolutionizing solid tumor treatment in the coming years.
To support research related to TCR-T therapies, we offer a wide range of TCR-related antigen MHC-antigen peptide complexes, including tissue differentiation antigens, cancer-testis antigens, mutation-associated neoantigens, and viral antigens. These complexes are available in monomer, tetramer, and fluorescent-labeled forms, as well as various HLA subtype backbones. They feature native conformations, excellent stability, and high bioactivity verified through flow cytometry, and they can specifically bind to corresponding TCRs with high affinity, fully supporting your TCR-T drug research process.
1. Shafer P, Kelly LM, Hoyos V. Cancer Therapy With TCR-Engineered T Cells: Current Strategies, Challenges, and Prospects. Front Immunol. 2022;13:835762. Published 2022 Mar 3. doi:10.3389/fimmu.2022.835762
2. Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci Adv. 2023;9(7):eadf3700. doi:10.1126/sciadv.adf3700
3. Tsimberidou AM, Van Morris K, Vo HH, et al. T-cell receptor-based therapy: an innovative therapeutic approach for solid tumors. J Hematol Oncol. 2021;14(1):102. Published 2021 Jun 30. doi:10.1186/s13045-021-01115-0
4. Liu Y, Yan X, Zhang F, et al. TCR-T Immunotherapy: The Challenges and Solutions. Front Oncol. 2022;11:794183. Published 2022 Jan 25. doi:10.3389/fonc.2021.794183
This web search service is supported by Google Inc.
