 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Application of Tau PFFs and Alpha-Synuclein PFFs in Neurodegenerative Disease Research 
The spread of prion-like protein aggregates is a key driver in neurodegenerative disease progression. In tauopathies and synucleinopathies, aggregation initiates locally through nucleation, subsequently propagating in a seed-mediated manner. Misfolded tau and α-synuclein (α-syn) form toxic filaments that are the main components for progression of Alzheimer's disease (AD) and Parkinson's disease (PD). Preformed fibrils (PFFs) of tau and α-syn serve as important tools for modelling abnormal protein aggregation and transmission. This study investigates the role of PFFs in cellular models, focusing on their involvement in aggregation, propagation, and potential applications in drug discovery.
https://doi.org/10.3389/fnins.2017.00064
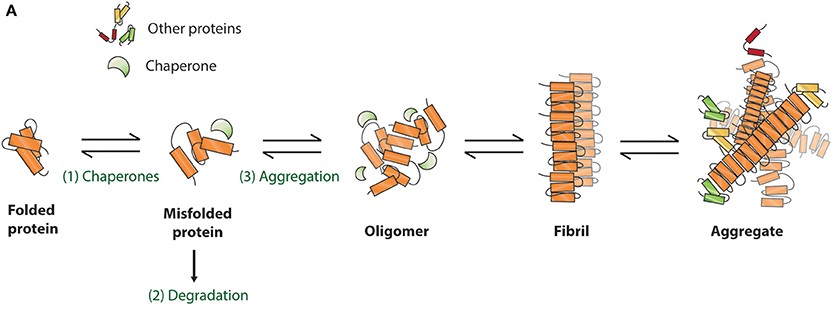
Schematic presentation of protein misfolding and aggregation
Neurodegenerative diseases like AD and PD are characterized by the abnormal aggregation and spread of misfolded proteins, tau and α-syn. In AD, tau protein becomes hyperphosphorylated and impairs its microtubule-binding function, leading to microtubule destabilization, neurofibrillary tangle formation, and neuronal death1. In PD, α-syn aggregates into Lewy bodies within nigrostriatal dopaminergic neurons, disrupting normal neuronal function and impairing synaptic transmission2. Tau and α-syn are both associated with prion-like self-propagation, where aggregates of misfolded proteins serve as templates, inducing pathological proteins to further misfold and spread throughout the brain3. As neuronal degeneration develops within the central nervous system (CNS), the accumulation and propagation of such misfolded proteins leads to autonomic dysfunction and cognitive and motor deficits.
PFFs of tau and α-syn are commonly used in models to study AD and PD progression. These fibrils serve as seeds to induce further aggregation in vitro and in vivo, mimicking disease progression and allowing for research into aggregation mechanisms and potential therapies4,5. This study evaluates the morphology of tau and α-syn PFFs before introducing them into cellular models to explore prion-like properties in neurodegenerative diseases.
Tau isoforms (0N3R, 0N4R, 1N3R, 1N4R, 2N3R, 2N4R) are classified based on amino-terminal exon inserts and carboxyl-terminal microtubule-binding domain (MBD) repeats6. Hexapeptide motifs in the MBD's repeat regions promote β-sheet formation, key to aggregation. Tau isoform expression is developmentally regulated, with 0N present in fetal brains and other isoforms in adult brains7. Aggregation of full-length tau is typically slow, so studies often use the aggregation-prone MBD construct K18 or the P301L mutation to enhance β-sheet formation8. α-syn isoforms, particularly α-syn-112 and α-syn-98, exhibit faster aggregation rates compared to the most abundant isoform, α-syn-1409. The A53T α-syn mutant increases neurotoxicity and is linked to early-onset PD10.
In this study, we prepared monomeric tau and α-syn for PFF formation. Following centrifugation, we collected the supernatant for protein concentration analysis and set aside a small portion of the monomers as negative controls. To induce PFF formation, we concentrated the protein solutions to 2-5 mg/mL and incubated them at 37°C with shaking for seven days. We then examined the morphological characteristics of wildtype and mutant tau and α-syn PFFs using transmission electron microscopy (TEM). Figure 1 shows the fibril morphology of tau-2N4R ( TAU-H5115), P301L mutant (TAU-H5113), α-syn-140 (ALN-H5115), and A53T mutant (ALN-H5114). Under negative stain TEM, fibrils ranged from 100 to 500 nm in diameter, with most appearing straight and displaying occasional twists. Despite varying aggregation patterns, both wild-type and mutant tau and α-syn fibrils formed thin and long networks.
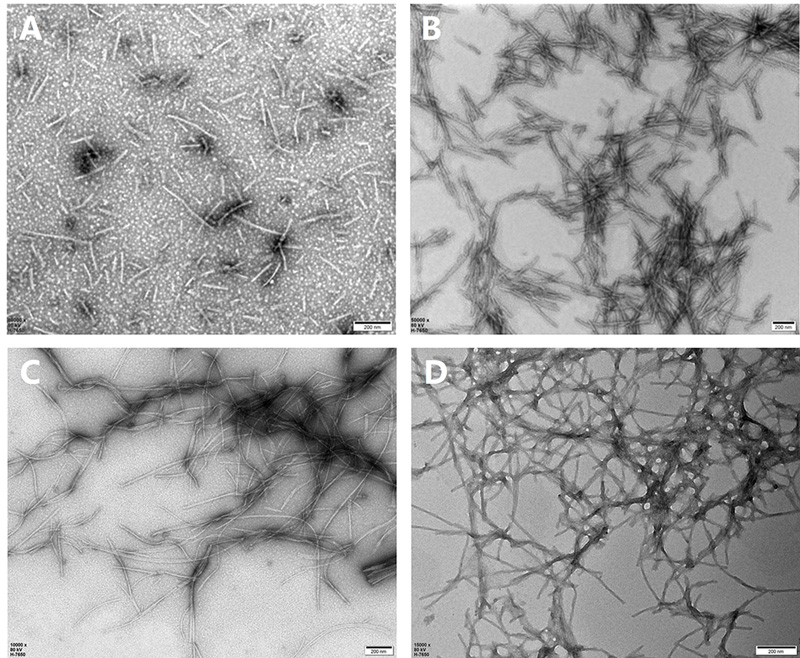
Figure 1. TEM images of tau and α-syn PFFs in wild-type and mutant forms. (A) tau WT PFFs (TAU-H5115); (B) Tau K18 P301L mutant PFFs (TAU-H5113); (C) α-syn WT PFFs (ALN-H5115); (D) α-syn A53T mutant PFFs (ALN-H5114). Scale Bar, 200 nm.
To investigate the aggregation kinetics of tau and α-syn PFFs, Thioflavin T (ThT) fluorescence assay was performed, which is sensitive to detect β-sheet-rich protein aggregates. In the tau ThT assay (Figure 2A), tau monomers (blue curve) showed slower aggregation, with fluorescence rising after 25 hours. In contrast, the addition of PFF (red curve) significantly accelerated the aggregation process, reducing the lag phase and increasing fluorescence intensity. Tau PFFs alone (orange-red curve) showed consistent fluorescence throughout the experiment, with no significant changes observed. In the α-syn ThT assay (Figure 2B), higher concentrations of α-syn PFFs led to faster aggregation (blue and green curves), indicated by shorter lag phases and increased fluorescence. α-syn monomers (yellow curve) showed a slower rise in fluorescence, reflecting delayed aggregation. The PBS control group in both tau and α-syn ThT assays exhibited no fluorescence increase, confirming the absence of aggregation. Therefore, the results demonstrate that both tau and α-syn PFFs significantly accelerated aggregation compared to their respective monomers.
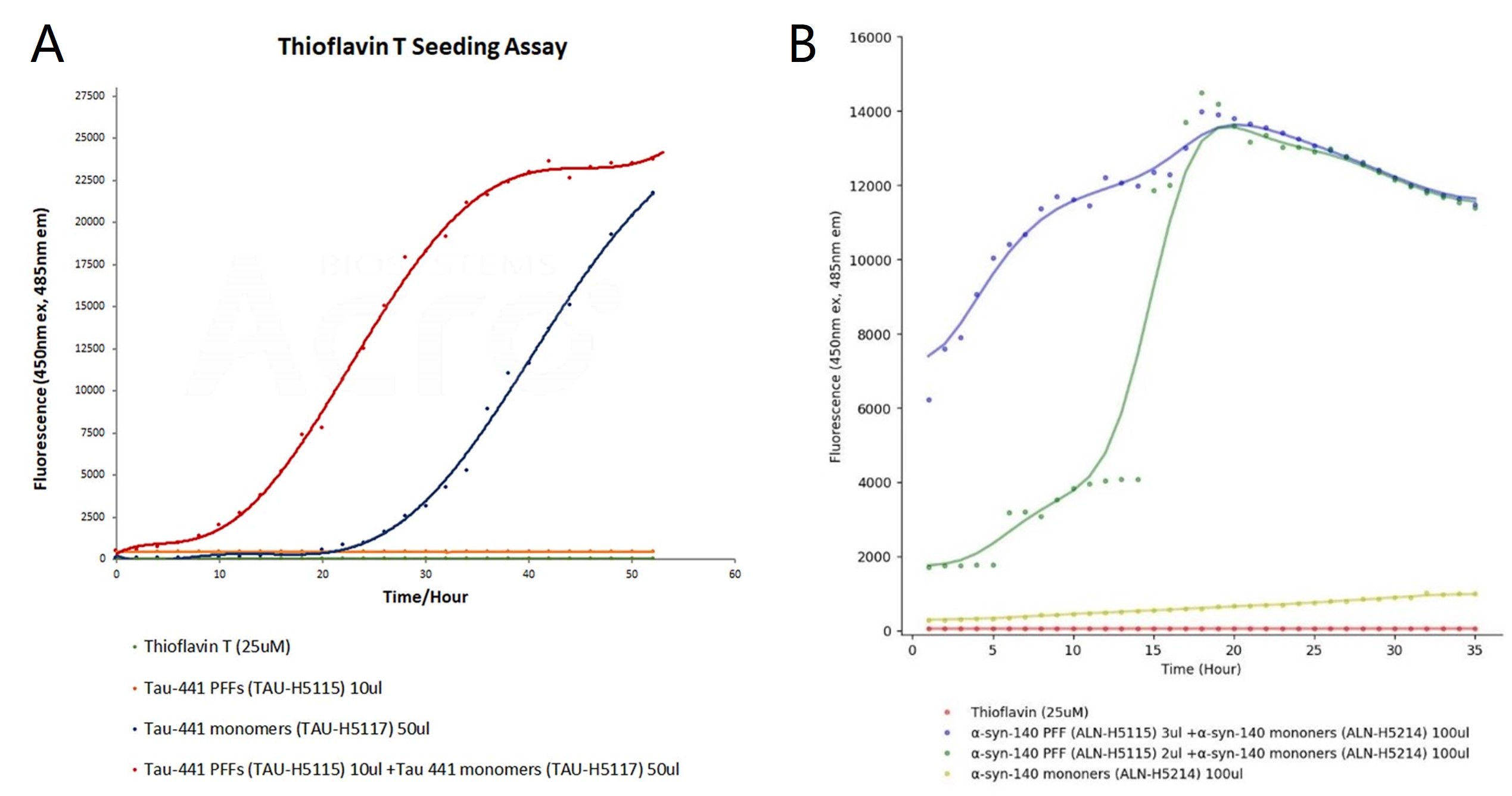
Figure 2. ThT seeding assay for tau and α-syn PFF. The emission curves show rising fluorescence over time, indicating aggregation when tau (TAU-H5117) and α-syn (ALN-H5214) monomers are added to tau PFFs (TAU-H5115) and α-syn PFFs (ALN-H5115), respectively.
To further investigate the pathology of tau and α-syn, stable HEK293 cell lines expressing GFP-tagged 2N4R tau (amino acids 244–372, CHEK-ATP087) and full-length GFP-tagged α-syn (CHEK-ATP085). These cells rarely form inclusions unless exposed to exogenous fibrils or prolonged passaging. After transfecting the cells with wild-type and mutant tau and α-syn PFFs, the formation of nuclear speckles and juxtanuclear inclusion bodies was observed using confocal microscopy. Moreover, Lipofectamine 2000 (Lipo2000)-mediated transduction enhanced the aggregation of both tau and α-syn protein, with mutant PFFs resulting in a greater number of inclusion bodies compared to wild-type (Figure 3A), suggesting that protein mutations increase aggregation and toxicity. The aggregated state is stably inherited, as cells with inclusion bodies tend to form localized clusters4. To evaluate whether the transfected cells exhibited similar behavior, we isolated and expanded cell lines containing tau and α-syn inclusion bodies in 96-well or 384-well plates. Quantified the proportion of inclusion body-positive cells using high-content microscopy. Our findings indicated that the inclusion body-positive morphology was maintained across multiple passages, demonstrating stable propagation of tau and α-syn aggregates (Figure 3B). This methodology successfully identified highly toxic cells with pronounced expression of inclusion bodies.
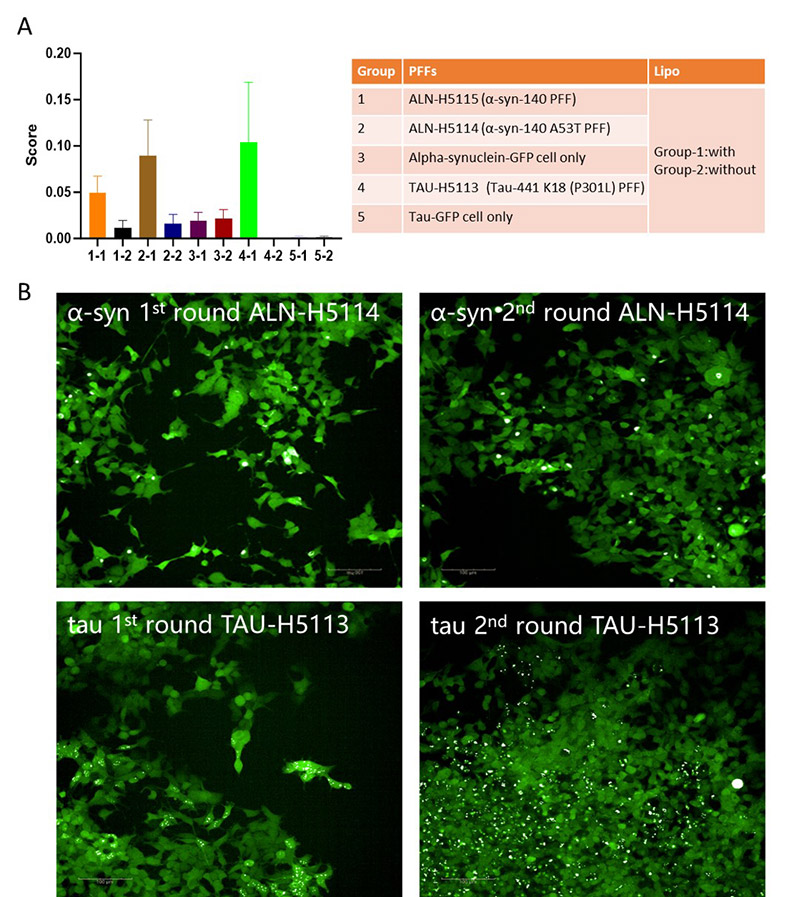
Figure 3. PFFs induce aggregation in cellular models. (A) Quantitative analysis of protein aggregation scores across different groups treated with PFFs of α-syn (ALN-H5114, ALN-H5115) and tau (TAU-H5113) or GFP controls, with and without lipid-based transfection agents. (B) Representative fluorescence images showing the progression of α-syn and tau aggregation in cellular models after two rounds of treatment with PFFs.
This study demonstrates that tau and α-syn PFFs effectively induce aggregation in HEK293 cell lines expressing GFP-tagged tau and α-synuclein, providing a model for studying neurodegenerative diseases like AD and PD. We observed that both wildtype and mutant PFFs promote aggregation, with mutant forms (tau P301L and α-syn A53T) leading to more aggressive pathology, consistent with their higher toxicity in clinical cases. Lipofectamine 2000 (Lipo2000) significantly enhanced the efficiency of PFFs transduction, increasing the aggregation of endogenous proteins. The stable inheritance of tau and α-syn aggregates across cell generations highlights the persistent nature of these aggregates and their potential role in disease progression. These findings underscore the utility of PFFs for studying protein aggregation mechanisms and for screening therapeutic compounds that target aggregation and its propagation. Further studies in more complex models, such as organoids and animal systems, are warranted to better replicate human disease conditions and advance our understanding of neurodegenerative disease pathogenesis.
Cell Culture
293T cells were passaged by pre-warming PBS, trypsin, and DMEM medium (10% FBS, 1% penicillin-streptomycin). After aspirating the culture medium, cells were rinsed with PBS and digested with trypsin at 37°C for 5 minutes. DMEM was added to stop digestion, and cells were centrifuged at 800 rpm for 3 minutes. The pellet was resuspended in DMEM and transferred to a new dish for continued culture.
PFFs Preparation
Tau and α-syn PFFs were prepared by diluting Tau and α-syn monomers to a concentration of 2-5 mg/ml. The protein solutions were incubated at 37°C with constant shaking for seven days. The quality and morphology of fibrils were assessed using TEM.
ThT assays
ThT (25 μM) was prepared in PBS and added to protein samples in a 96-well plate (100 μL per well). PFFs (9 μM) or monomers (120 μM) (or both) were incubated at 37°C, 200 rpm. Measure fluorescence using a CLARIOstar plate reader at 450 nm excitation and 485 nm emission every 1-72 hours to track aggregation.
PFFs Transduction
Cells were transduced with PFFs using Lipofectamine 2000 (Lipo2000, Invitrogen). 100 μL Opti-MEM containing 8 μL Lipo2000 was mixed with an equal volume of sonicated PFFs and incubated for 25 minutes at room temperature. The mixture was then added to the cells in a 12-well plate and incubated at 37°C with 5% CO₂ for 48 hours.
Aggregation Detection
After transduction, cells were washed with PBS and fixed with 4% paraformaldehyde for 15 minutes. Aggregation of GFP-tagged tau and α-syn was detected using confocal microscopy. The number and morphology of inclusions were quantified by high-content microscopy.
1. Rawat, P., Sehar, U., Bisht, J., Selman, A., Culberson, J., and Reddy, P.H. (2022). Phosphorylated Tau in Alzheimer's Disease and Other Tauopathies. Int J Mol Sci 23. 10.3390/ijms232112841.
2. Cookson, M.R. (2009). alpha-Synuclein and neuronal cell death. Mol Neurodegener 4, 9. 10.1186/1750-1326-4-9.
3. Jan, A., Gonçalves, N.P., Vaegter, C.B., Jensen, P.H., and Ferreira, N. (2021). The Prion-Like Spreading of Alpha-Synuclein in Parkinson's Disease: Update on Models and Hypotheses. Int J Mol Sci 22. 10.3390/ijms22158338.
4. Kaufman, S.K., Sanders, D.W., Thomas, T.L., Ruchinskas, A.J., Vaquer-Alicea, J., Sharma, A.M., Miller, T.M., and Diamond, M.I. (2016). Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 92, 796-812. 10.1016/j.neuron.2016.09.055.
5. Sanders, D.W., Kaufman, S.K., DeVos, S.L., Sharma, A.M., Mirbaha, H., Li, A., Barker, S.J., Foley, A.C., Thorpe, J.R., Serpell, L.C., et al. (2014). Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271-1288. 10.1016/j.neuron.2014.04.047.
6. Peng, C., Wei, W., Zhang, H., Wang, Y., Chang, B., Zhao, W., Jia, L., Li, L., Lu, F., and Liu, F. (2024). Heterologous expression and fibrillary characterization of the microtubule-binding domain of tau associated with tauopathies. Molecular Biology Reports 51, 184. 10.1007/s11033-024-09231-z.
7. Bachmann, S., Bell, M., Klimek, J., and Zempel, H. (2021). Differential Effects of the Six Human TAU Isoforms: Somatic Retention of 2N-TAU and Increased Microtubule Number Induced by 4R-TAU. Front Neurosci 15, 643115. 10.3389/fnins.2021.643115.
8. Elbaum-Garfinkle, S., Cobb, G., Compton, J.T., Li, X.-H., and Rhoades, E. (2014). Tau mutants bind tubulin heterodimers with enhanced affinity. Proceedings of the National Academy of Sciences 111, 6311-6316. doi:10.1073/pnas.1315983111.
9. Röntgen, A., Toprakcioglu, Z., Tomkins, J.E., and Vendruscolo, M. (2024). Modulation of α-synuclein in vitro aggregation kinetics by its alternative splice isoforms. Proc Natl Acad Sci U S A 121, e2313465121. 10.1073/pnas.2313465121.
10. Polymeropoulos, M.H., Lavedan, C., Leroy, E., Ide, S.E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045-2047. 10.1126/science.276.5321.2045.
This web search service is supported by Google Inc.
