Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > One jab and you’re done! CanSino and Johnson & Johnson adenovirus vaccines receive regulatory approval. On February 25, the Chinese NMPA official website announced that the State Food and Drug Administration conditionally approved the CanSino biological AG adenoviral vaccine to market. This is the third COVID-19 vaccine approved for marketing in China and the first approved adenovirus vector-based vaccine. Prior to this, CanSino had obtained emergency use authorizations (EUAs) from Pakistan and Mexico. Within days, the FDA unanimously granted EUA to Johnson & Johnson’s vaccine for people over the age of 18. This is the third new COVID-19 vaccine approved in the United States and the first approved adenovirus type 26 vector-based vaccine.

Figure 1. Johnson & Johnson application presented to the FDA expert panel
These two adenovirus vector-based vaccines require only one shot to provide protection which will vastly simplify vaccination campaign compared to two shot regiment needed for inactivated and mRNA based vaccines. Additionally, the adenovirus vaccine does not require cryogenic temperature for storage enabling vaccination campaigns in developing countries.
According to the published Phase 3 clinical trials provided by CanSino, the vaccine protected 65.28% patients against mild symptoms 28 days after single injection, while the protective effect against severe illness 14 days after injection was determined to be 90.07%. The overall protective efficacy of the vaccine is 68.83% and 95.47% for mild and severe cases respectively.
Johnson and Johnson’s FDA application also disclosed their phase III data. The phase III clinical trial included 44,325 participants worldwide of which 47% were from the United States, 40% were from Latin America (17% Brazilian variant), and 13% from South Africa. Of all the participants 24% are seniors over 65 and 40% have at least one chronic underlying disease. After 14 days from injection, the vaccine provided protection to 66.9% of patients against moderate to critical cases of COVID-19. 28 days after receiving the single injection, the protection effect stayed unchanged at 66.1%. This vaccine was also shown to be effective for the elderly and little difference in efficacy among people with underlying diseases and young participants without underlying diseases was observed. Furthermore, Johnson & Johnson also presented data on protective effect of this vaccine against South Africa and Brazilian variants. In the United States, where the original D614G strain is dominant, the vaccine efficacy is 72% (severe symptom protection 86%). In South Africa, where the B.1.351 variant strain account for 95% of all infections, the vaccine efficacy dropped to 64% (severe symptom protection 82%). Against Brazilian P.2 variant, where the strains account for nearly 70% of all infections, the vaccine efficacy is 68% (severe symptom protection 88% ) (Figure 2 ). Overall, the Johnson & Johnson's vaccine displays strong protection against both South African and Brazilian variant comparable to the original strain. This finding is a good news particularly due to concerns of resurgence of cases due to immune escape of the variants.
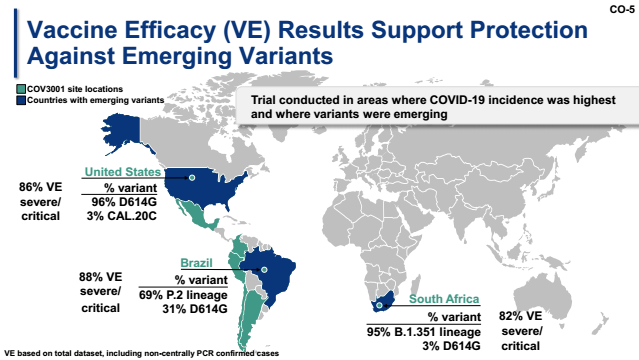
Figure 2. Summary of the protective effect of Johnson & Johnson’s vaccine on the new coronavirus mutant strain
The approval of CanSino and Johnson & Johnson's vaccines adds to the arsenal against COVID-19 particularly with the positive data against variants.
As part of the vaccine effectiveness data, Johnson and Johnson presented neutralizing antibody titers produced in the human body 14 days and 28 days after vaccination. Neutralizing antibody is a type of specific antibody produced in immune response that blocks pathogens from invading host cells. Therefore, the presence of neutralizing antibodies in vaccinated sera translates to the clinical protective efficacy of the vaccine.
At present, the gold standard neutralizing antibody detection is Plaque Reduction Neutralization Titer (PRNT) assay based on live viruses and cells performed in the P3 laboratory setting. Due to the complexity, requirement and slow throughput of the assay, surrogate neutralization assay is commonly used to detect neutralizing antibodies. These neutralization assay can be readily used in assessing neutralizing antibody titer of vaccinated population. At present, the main methods used to detect neutralizing antibodies are chemiluminescence, colloidal gold and enzyme-linked immunoassays (ELISA) each with its own unique advantages. ELISAs can be used for qualitative and semi-quantitative detection of neutralizing antibodies with high sensitivity and high throughput. Currently, the only FDA approved SARS-CoV-2 neutralizing antibody detection kit is an ELISA. Experimental data shows that it is highly correlated to neutralizing antibody titers using real and pseudovirus based PRNT assay.
> > > > Click to see more evaluation of core vaccine reagent
> > > > Click for more diagnostic material
The addition of two new vaccines from CanSino and Johnson and Johnson provides optimism in lowering SARS-CoV-2 infection and COVID-19 cases by the end of the year. The current burden of SARS-CoV-2 is most acutely faced by North and South America and Europe followed by South East Asia according to the CDC.
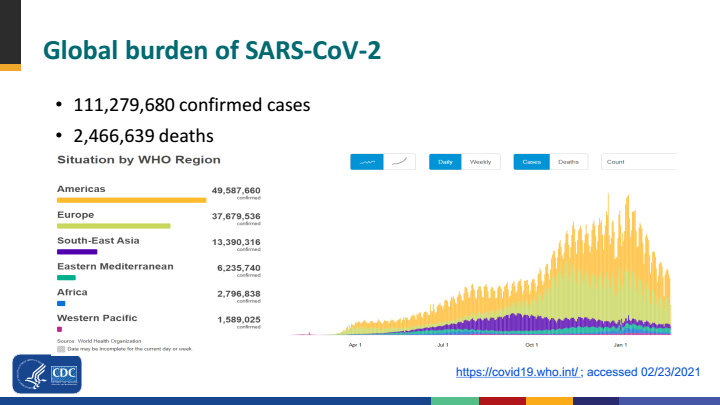
Figure 3. CDC data on global trend of COVID-19 pandemic
However,
the emerging SARS-CoV-2 variant presents a high risk of pandemic
resurgence and needs to be assessed during vaccine and therapeutic
development.
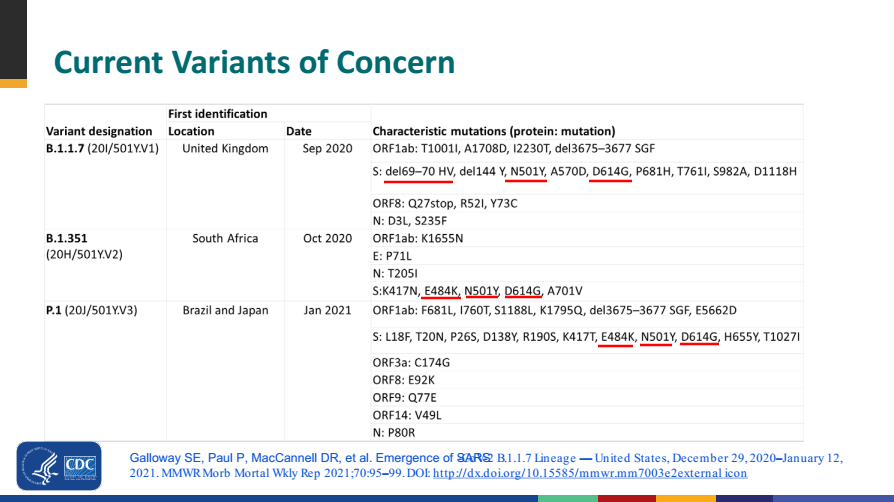
Figure 4 Key mutation sites of interest
ACROBiosystem is closely tracking these emerging variants and offers S trimer, RBD and NTD mutants based on the three leading variants with variety of tags. Single S protein mutants are also available for ordering.
Click to check more variant proteins information.
This web search service is supported by Google Inc.