 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
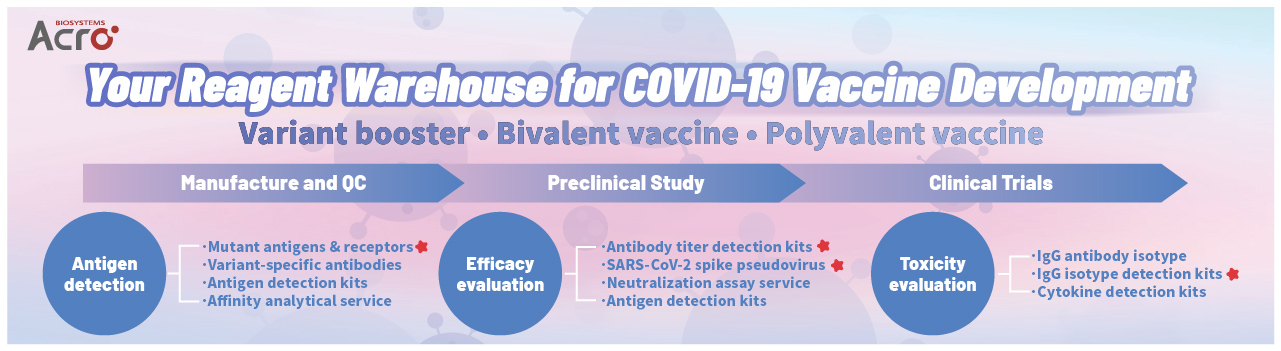
> Your Reagent Warehouse for COVID-19 Vaccine Development
Since December 2019, the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated disease, COVID-19, has caused a devastating pandemic worldwide. The SARS-CoV-2 pandemic presents an extraordinary challenge to the lives and health of the global population, resulting in a total of over 200 million diagnosed cases and 4.9 million death (as of October 2021). It also significantly affected society, economics and the environment around the world and changed everyone's life.
As the virus spreads globally, the continuous emergence of new mutant strains escalated the challenge on humans. To better track and inform the viral variants, the World Health Organization (WHO) characterized several variants as "Variants of Concern (VOCs) " and "Variants of Interest (VOIs) " based on the risk posed to global public health. Today, of hundreds of variants that evolved from the original wild-type virus, Delta (PANGO lineage B.1.617.2/AY) are dominantly circulating and shaping the trend, reported to be responsible for most of the cases worldwide.
Vaccination is the cornerstone to prevent and control the spread of infectious diseases. Despite the importance of mask-wearing and social distancing, immunization by vaccines remains the most cost-effective way to protect our health. For this reason, vaccine development and production are at the center of each nation's public health policy.
ACROBiosystems is a life science company that provides reagents for the global vaccine industry. Having mobilized forces to develop SARS-CoV-2 recombinant antigens since the pandemic and accumulated mature technology and experiences with the virus and vaccines, we supply 200+ products and customized services that cover all the dominant strains of SARS-CoV-2 and support vaccine development of all technological platforms.
>>> Download the poster about core research tools for COVID-19 vaccine R&D
During the special time of need, we will continue to track the epidemic and are determined to facilitate the trial and application of the vaccines.
In more than a year after the virus outbreak, various scientific research units and vaccine companies have worked to successfully develop a variety of COVID-19 vaccines. The vaccines can be classified by different technological platforms into whole virus vaccines, subunit vaccines, viral vector vaccines and gene vaccines (Fig. 1). Currently, 7 vaccines have obtained the WHO's emergency use authorization to be used in massivevaccination on a global scale, and over 300 vaccines are under development in pre-clinical or clinical trial stage.
Whole virus/Protein based:SDS-PAGE, MS, ELISA;
Virus vectored/ Nucleic acid:Western Blot, ELISA, FACS;
Protein based:ELISA, SPR, BLI;
IgG titer detection:ELISA Neutralizing Ab titer detection cVNT, pVNT, sVNT.
①In vivo efficacy evaluation:
★Humoral immunity:
ⅠMouse/Monkey IgG titer detection
ⅡNeutralizing Ab titer detection
★Cellular immunity:Elispot
①Antigen Detection
①In vivo efficacy evaluation:
★Humoral immunity:
ⅠHuman IgG titer detection
ⅡNeutralizing Ab titer detection
★Cellular immunity:Elispot
②In vitro efficacy evaluation:Antigen Detection
①ADE/VED detection:IgG isotype titer mesurement
>>> Development and Licensure of Vaccines to Prevent COVID-19
Super stable SARS-CoV-2 spike trimer
RBD/S trimer antigen quantitative detection kit
Variants specific antibodies 
The ideal control for antigen detection:Super stable SARS-CoV-2 spike trimer
| Lineage | Cat. No. | Mutation | Tag | Preorder/Order |
|---|
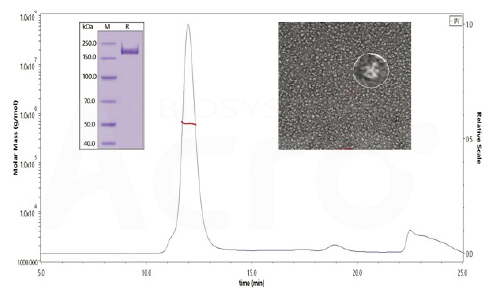
The purity of SARS-CoV-2 S protein, His Tag, Super stable trimer (Cat. No. SPN-C52H9) is more than 90% verified by SDS-PAGE under reducing (R) condition. The molecular weight was around 550-660 kDa confirmed by SEC-MALS. The particles are similar in size and appearance to SARS-CoV-2 trimers reported in published literature verified by negative stain electron micrography.
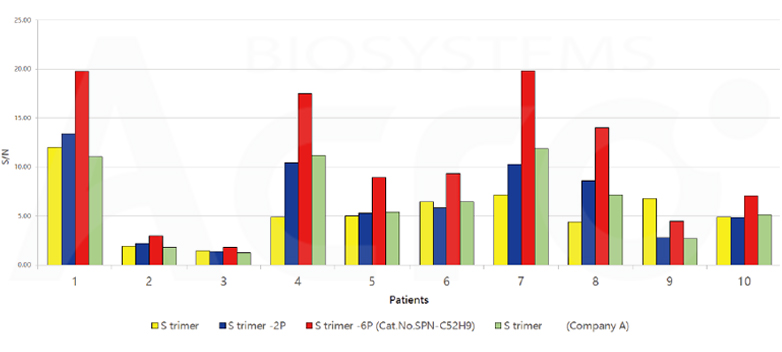
SARS-CoV-2 S protein, His Tag, Super stable trimer (Cat. No. SPN-C52H9) shows highest signal-to-noise ratio as compared to other spike proteins constructs in antibody tests in convalescent serum
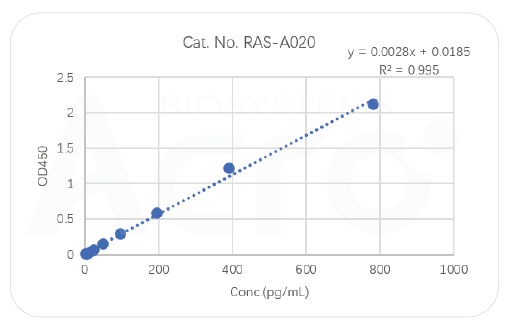
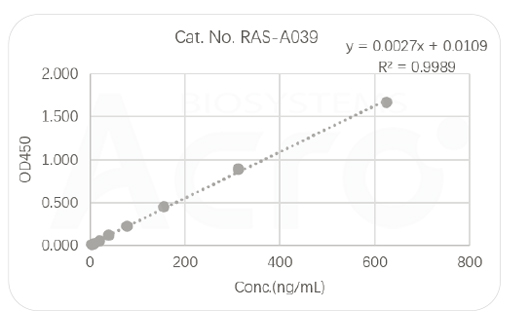
Highly efficient tools for antigen detection:RBD/S trimer antigen quantitative detection kit (sandwich ELISA)
| Cat. No. | Product Description | Preorder/Order |
|---|
For mutant antigen detection in next-generation Vaccine R&D: Omicron/Delta/Beta & Gamma specific antibodies
| Cat. No. | Isotype | Product Description | Epitope | Preorder/Order |
|---|
Loaded Monoclonal Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1 (Cat. No. SPD-M305) on AMC Biosensor, can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) with an affinity constant of 9.07 nM as determined in BLI assay (ForteBio Octet Red96e).
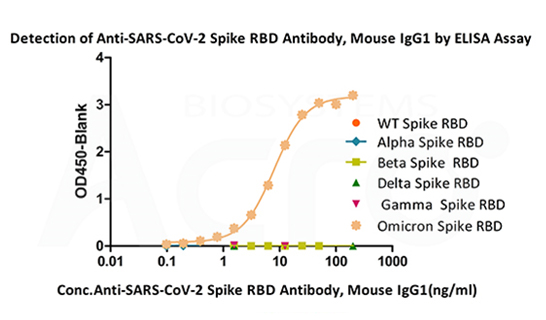
Immobilized SARS-CoV-2 Spike RBD (Omicron, Cat. No. SPD-C522e) can bind Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1(Cat. No.SPD-M305) with a linear range of 0.4-12.5 ng/mL. The antibody does not bind Spike RBD of WT (Cat. No. SPD-C52H1), Alpha (Cat. No. SPD-C52Hn), Beta (Cat. No. SPD-C52Hp), Delta(Cat. No. SPD-C52Hh) and Gamma (Cat. No. SPD-C52Hr).
>>> The activity of antibodies were verified by pseudovirus. Click to learn more.
ACE2 and other host receptors
Antigen-ACE2 interaction service
High quality dimeric receptor:ACE2 proteins
| Molecule | Cat. No. | Species | Tag | Host | Product Description | Preorder/Order |
|---|
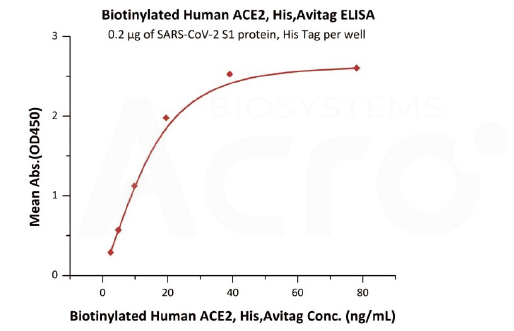
Immobilized SARS-CoV-2 S1 protein, His Tag (Cat. No. S1N-C52H3) at 2 μg/mL (100 μL/well) can bind Biotinylated Human ACE2, His,Avitag (Cat. No. AC2-H82E6) with a linear range of 2-20 ng/mL (QC tested).
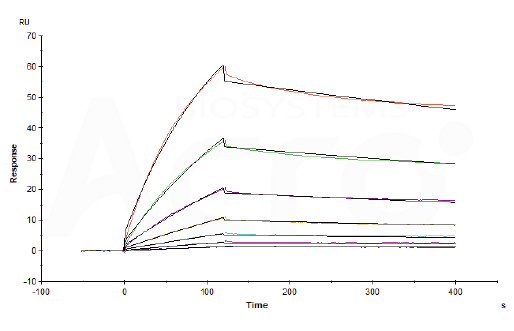
Human ACE2, Fc Tag (Cat. No. AC2-H5257) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind recombinant SARS-CoV-2 Spike vaccine with an affinity constant of 16.9 nM as determined in a SPR assay (Biacore T200).
Aid comprehensive research on antigen-ACE2 interaction:Molecular interaction analytical services
Total Antibody Titer Determination
Neutralizing Antibody Titer Detection
Spike protein pseudovirus
Broad-spectrum anti-RBD neutralizing antibody
In vivo antibody titer determination in preclinical & clinical trial:S trimer/RBD/S1 IgG Antibody Titer Detection Kit (indirect ELISA)
| Cat. No. | Epitope | Product Description | Preorder/Order |
|---|
| Cat. No. | Epitope | Product Description | Preorder/Order |
|---|
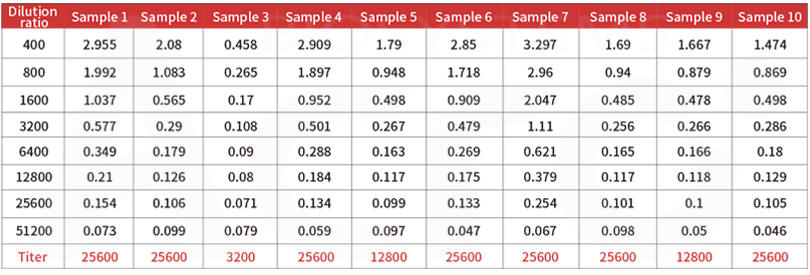
Post-vaccination serum samples are tested with Anti-SARS-CoV-2 Antibody IgG Titer Serologic Assay Kit (Spike RBD) (Cat. No.RAS-T024), which accurately and precisely measure antibody titer in serum (Accuracy≤±15%; Intra-assay precision <10%; Inter-assay precision <15%).
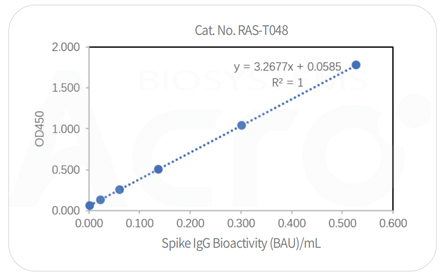
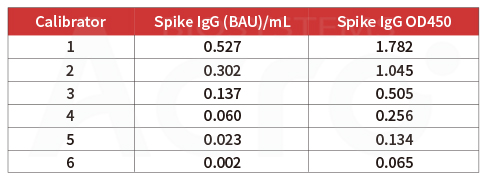
Anti-SARS-CoV-2 Antibody IgG Quantitative Detection Kit (Spike Trimer) (Cat. No. RAS-T048) is developed for quantitative detection of IgG antibodies in post-vaccination serum. This kit is an important supplement to the existing vaccine evaluation methods which significantly improve the efficiency and accuracy of vaccine evaluation.
High-throughput alternative for neutralizing antibody detection: Neutralizing Antibody Titer Detection Kit (competitive ELISA)
| Lineage | Cat. No. | Product Description | Preorder/Order |
|---|
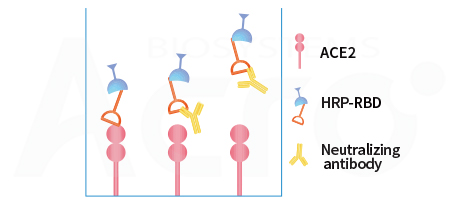
Competitive ELISA
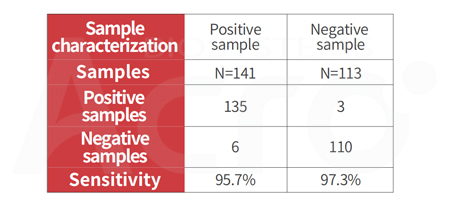
Post-vaccination serum NAb test (Cat. No. RAS-N022) demonstrates high sensitivity and accuracy

16 convalescent serum samples were tested for neutralizing antibody level with pseudovirus and ELISA kit (Cat. No. RAS-N022), respectively. The results are highly correlated (R2 =93.73)。
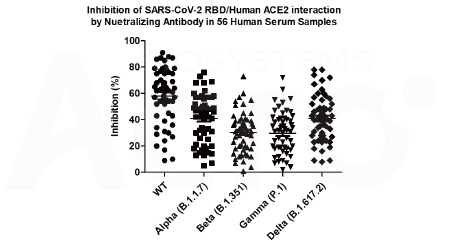
56 post-vaccination (inactivated vaccines) serum samples are tested for neutralizing capacity against multiple strains (Alpha, Beta, Gamma, Delta) of SARS-CoV-2 with wild type (WT) and mutant-specific kits. Neutralization against all VOCs is lower than the WT, with Delta being the lowest.
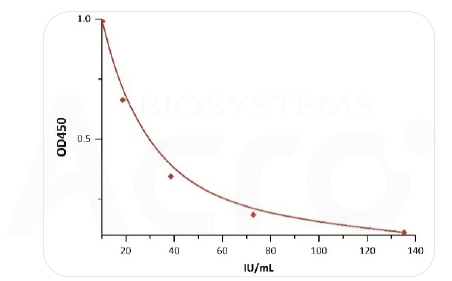
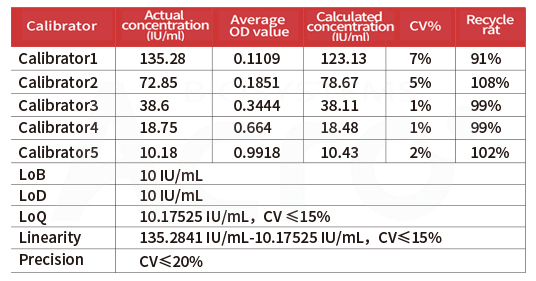
Note:y = (A - D) / [1 + (x/C)^B] + D (A =1.55562 B =1.45522 C=14.42024 D=0.05532 r^2= 1)
Safe and approved neutralizing antibody detection method: Spike protein pseudovirus
| Cat. No. | Product Description | Preorder/Order |
|---|
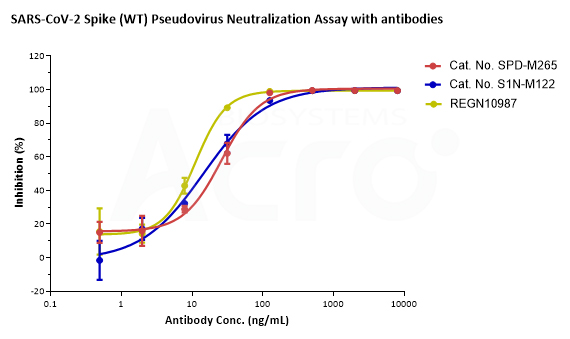
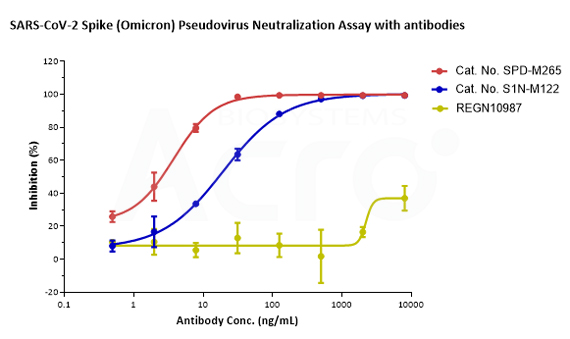
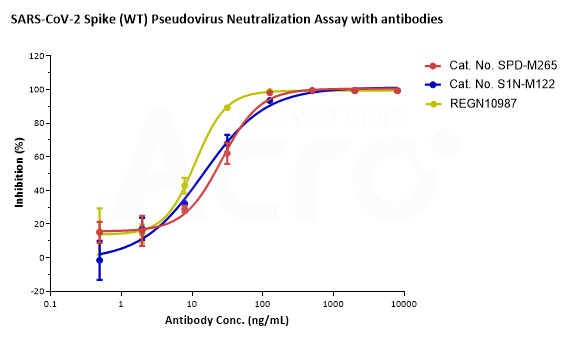
SARS-CoV-2 Spike (WT) Fluc-GFP Pseudovirus (Cat. No. PSSW-HLGB001) and SARS-CoV-2 Spike (Omicron) Fluc-GFP Pseudovirus (Cat. No. PSSO-HLGB003) can be neutralized by Anti-SARS-CoV-2 Spike RBD Neutralizing Antibody (Cat. No. SPD-M265, S1N-M122) with inhibition rates of more than 99%. The antibody, REGN10987, which has been reported that Omicron mutant strain can escape, can effectively neutralize SARS-CoV-2 Spike (WT) Fluc-GFP Pseudovirus (Cat. No. PSSW-HLGB001) but not SARS-CoV-2 Spike (Omicron) Fluc-GFP Pseudovirus (Cat. No. PSSO-HLGB003).
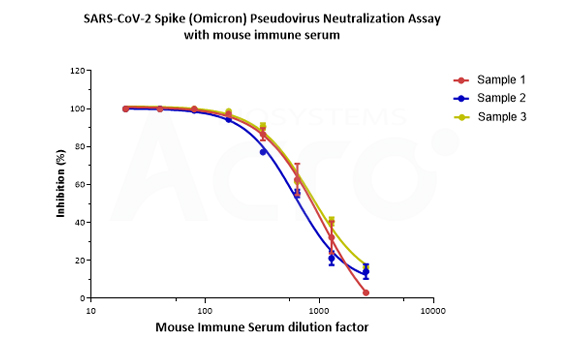
SARS-CoV-2 Spike (Omicron) Fluc-GFP Pseudovirus (Cat. No. PSSO-HLGB003) can be neutralized by serum from immunized mice. The inhibition rates come up to more than 99%.
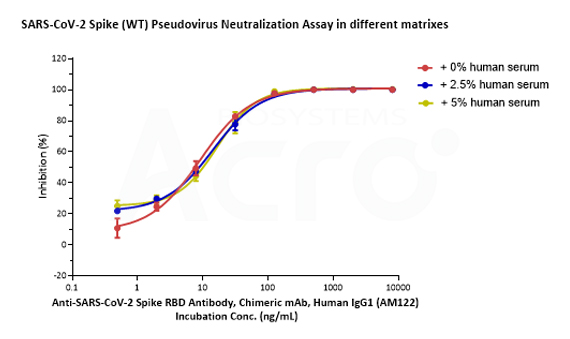
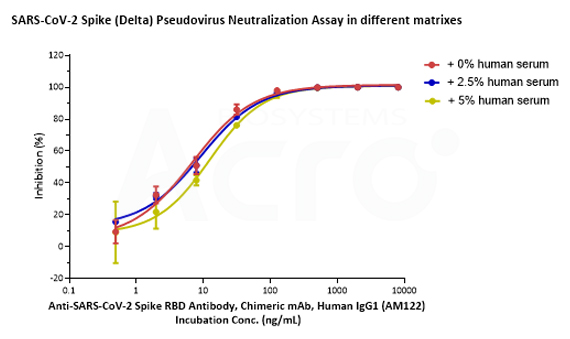
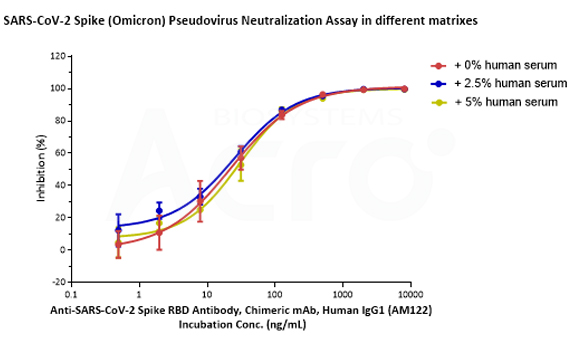
SARS-CoV-2 Spike (WT) Fluc-GFP Pseudovirus (Cat. No. PSSW-HLGB001), SARS-CoV-2 Spike (Delta) Fluc-GFP Pseudovirus (Cat. No. PSSD-HLGB002) and SARS-CoV-2 Spike (Omicron) Fluc-GFP Pseudovirus (Cat. No. PSSO-HLGB003) can be neutralized by Anti-SARS-CoV-2 Spike RBD Neutralizing Antibody (Cat. No. S1N-M122) with similar dose-effect relationship in various matrixes (culture medium with 0%, 2.5% or 5% human negative serum).
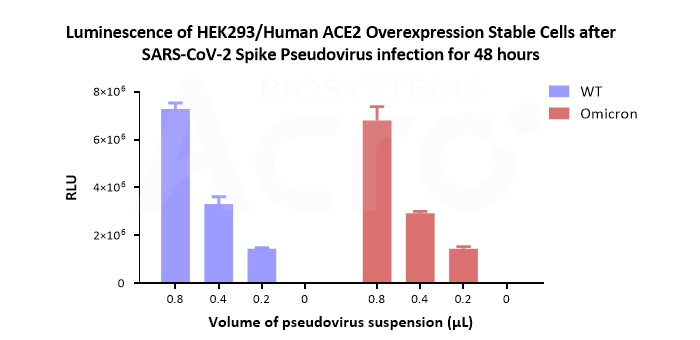
SARS-CoV-2 Spike (WT) Fluc-GFP Pseudovirus (Cat. No. PSSW-HLGB001) and SARS-CoV-2 Spike (Omicron) Fluc-GFP Pseudovirus (Cat. No. PSSO-HLGB003) both hold high titer. The luminescence value after the pseudovirus infect 5×104 human ACE2 overexpression cells (Cat. No. CHEK-ATP042) for 48 h come up to more than 106. The luciferase reporter is suitable for quantitative experiments.
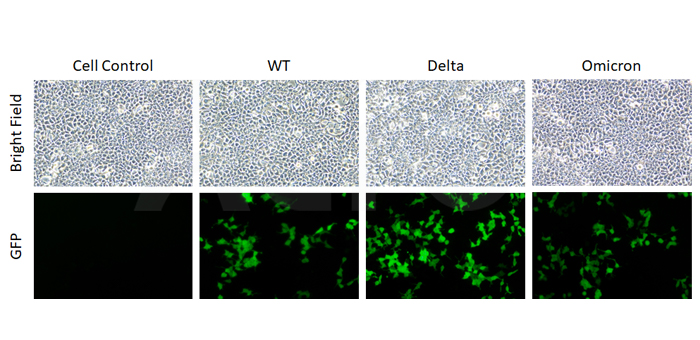
SARS-CoV-2 Spike (WT) Fluc-GFP Pseudovirus (Cat. No. PSSW-HLGB001), SARS-CoV-2 Spike (Delta) Fluc-GFP Pseudovirus (Cat. No. PSSD-HLGB002) and SARS-CoV-2 Spike (Omicron) Fluc-GFP Pseudovirus (Cat. No. PSSO-HLGB003) all contain GFP reporter. The GFP reporter is used for imaging.
Qualified positive control for neutralizing antibody detection:Broad-spectrum anti-RBD neutralizing antibody
| Cat. No. | Species | Product Description | Isotype | Preorder/Order |
|---|
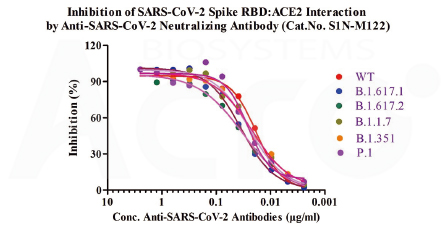
Anti-SARS-CoV-2 Spike RBD Neutralizing antibody (Cat. No.S1N-M122)neutralizes SARS-CoV-2 Spike RBD by inhibiting RBD: ACE2 interaction. The ACE2-coated plate is incubated with the wild type (WT) RBD or B.1.1.7, B.1.351, P.1, B.1.617.1, B.1.617.2 mutant and treated with the neutralizing antibody at increasing concentration. Percent inhibition is calculated based on the OD value.
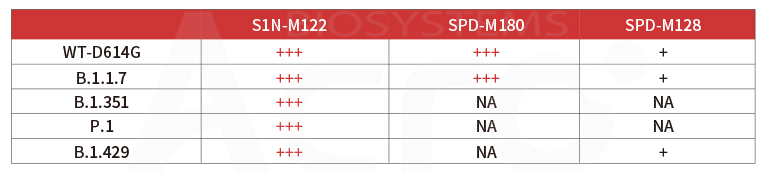
As verified by SARS-CoV-2 spike pseudovirus neutralization test, the broad-spectrum neutralizing antibody (Cat. No. S1N-M122) can effectively neutralize wild-type and Alpha (B.1.1.7) /Beta (B.1.351) /Gamma (P.1) /Delta (B.1.617.2, data not shown) viral mutants, making it suitable as a quality control antibody in neutralizing antibody screening for mutant strains, neutralizing antibody detection in convalescent or vaccinated serum, etc.
antibody subtype detection
IgG Antibody Subtype Detection Kit (indirect ELISA):support research in ADE and ERD
| Lineage | Cat. No. | Product Description | Preorder/Order |
|---|
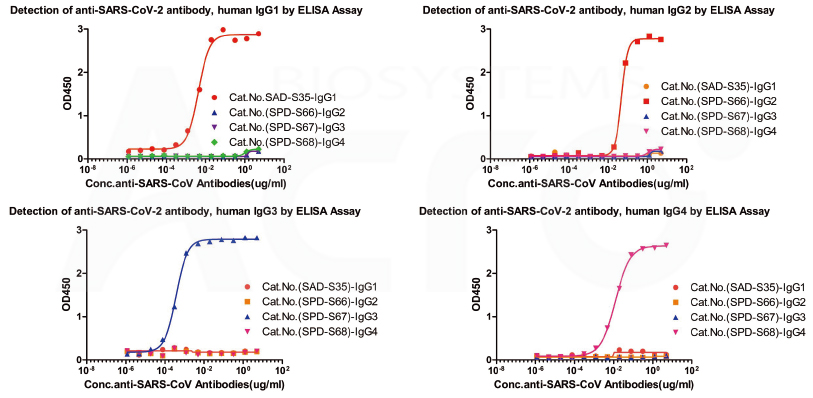
Cross-validation data for antibody subtype detection
Authors: Sasisekharan, Varun et al.
Journal: Proc Natl Acad Sci U S A 2021
Cited Product: SPD-C52H3 ,NUN-C5227
Application: ELISA
Authors: Esparza, Thomas J et al.
Journal: Scientific reports 2020
Cited Product: S1N-C5255,AC2-H52H8,SPD-C52H3,SPD-C52H3,SPD-C82E9,AC2-H82E6,SPD-S52H7,SPD-S52H4
Application: Immunization, Competitive Immunopanning, SPR & Binding Assay & Phage Display Clone Screening, BLI, Binding Assay
Antibody Signature Induced by SARS-CoV-2 Spike Protein Immunogens in Rabbits
Authors: Ravichandran S, Coyle EM, Klenow L, et al.
Journal: Sci Transl Med 2020
Cited Product: AC2-H52H8
Application: SPR
Antibody-like proteins that capture and neutralize SARS-CoV-2
Authors: Kondo T, Iwatani Y, Matsuoka K, et al.
Journal: Science advances 2020
Cited Product: S1N-S52H5
Application: ELISA
Binding of the SARS-CoV-2 Spike Protein to Glycans
Authors: Wei Hao,et al
Journal: BioRxiv (preprint) 2020
Cited Product: S1N-C52H3
Application: glycan microarray
Authors: Yan Lou. et al
Journal: BioRxiv (preprint) 2020
Cited Product: SPD-S52H4SPD-S52H7,SPD-S52H8
Application: BLI,ELISA
Authors: Tyler N Starr, Allison J Greaney , Sarah K Hilton, et al.
Journal: BioRxiv (preprint) 2020
Cited Product: AC2-H82E6
Application: Flow cytometry
Flow-Mediated Susceptibility and Molecular Response of Cerebral Endothelia to SARS-CoV-2 Infection
Authors: Kaneko N, Satta S, Komuro Y, et al.
Journal: Stroke 2020
Cited Product: S1N-C82E8
Application: ELISA
HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry
Authors: Wei C, Wan L, Yan Q, et al.
Journal: Nature metabolism 2020
Cited Product: SPN-C52H9,S1N-C52H3,S2N-C52H5,SPD-C52H3
Application: SPR & FACS & Confocal microscopy
Immune responses to SARS-CoV-2 in three children of parents with symptomatic COVID-19
Authors: Tosif S, Neeland MR, Sutton P, et al.
Journal: Nature communications 2020
Cited Product: S1N-S52H5,S2N-C52H5,S1N-C52H3
Application: ELISA\FACS
Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults
Authors: Mulligan, Mark J et al.
Journal: Nature 2020
Cited Product: SPD-C82E9
Application: Immunogenicity assessments
SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden
Authors: Rudberg AS, Havervall S, Månberg A, et al.
Journal: Nat Commun 2020
Cited Product: NUN-C5227
Application: Luminex
Authors: Luetkens T, Metcalf R, Planelles V, et al.
Journal: Blood Adv 2020
Cited Product: S1N-C52H3
Application: ELISA
This web search service is supported by Google Inc.
