Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> high-throughput solutions for vaccine R&D based on ELISA

Vaccination is one of the most important and economic tools to protect the community from infectious diseases. There are about 60% of the population were fully vaccinated around the world, however, as the virus mutates continuously, the increasingly strong transmission and immune escape capabilities of the mutant strains have brought great challenges to the prevention and control of the epidemic. The emergence of many breakthrough infection cases represents that the "immune barrier" created by vaccination against the wild-type strain of COVID-19 may be flawed. Therefore we urgently need next-generation vaccines that still have high protective efficacy against mutant strains.
As the world experiences a new wave of outbreaks due to the emergence of the Omicron variant, vaccine manufacturers have already stepped up for the development of vaccines against new variants. Earlier, Moderna and Pfizer had announced to start the research on candidate vaccines against Delta and Omicron variants.
In order to support and regulate the development of COVID-19 vaccines, the Food and Drug Administration (FDA) issued the "Development and Licensure of Vaccines to Prevent COVID-19" to determine key indicators for monitoring the whole process of vaccine development.
>>> click to download the Development and Licensure of Vaccines to Prevent COVID-19
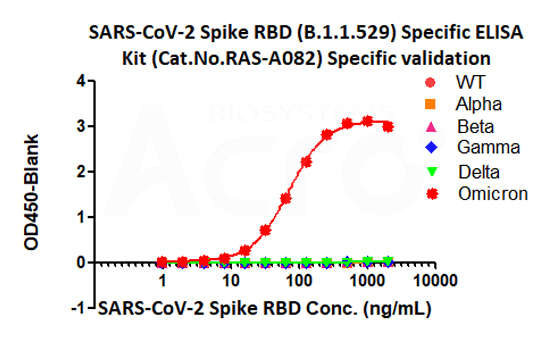
ACROBiosystems has undergone methodological validation and independently developed a series of COVID-19-related ELISA kits according to the application as described in the guidelines, aiming to provide comprehensive solutions ranging from quality control of vaccine production to preclinical and clinical research.
| Cat. No. | Product Description | Preorder/Order |
|---|
| Lineage | Cat. No. | Product Description | Preorder/Order |
|---|
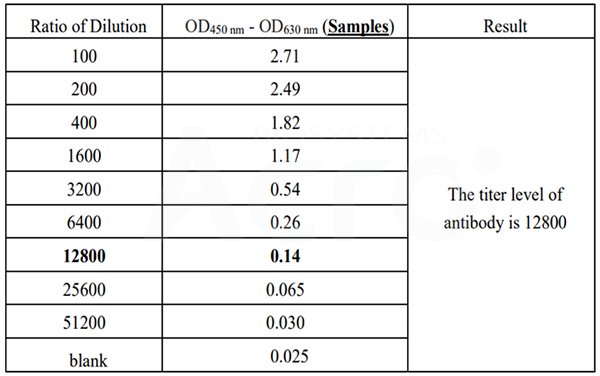
Cat. No. RAS-T045 can be used to detect the mouse IgG antibody titer, the titer value is 12800.
| Lineage | Cat. No. | Product Description | Preorder/Order |
|---|
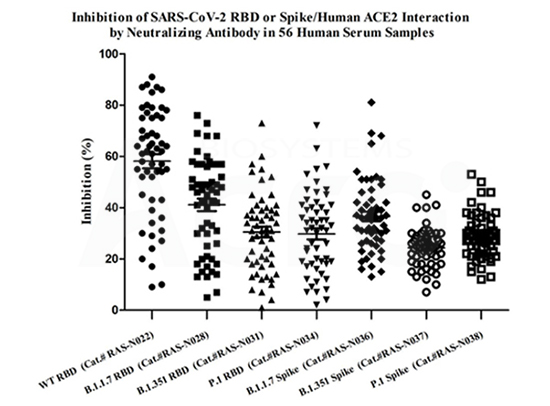
Anti-SARS-COV-2 Neutralizing Antibody Titer Serologic Assay Kit based on WT RBD (Cat. No. RAS-N022), B.1.1.7 RBD (Cat. No. RAS-N028), B.1.351 RBD (Cat. No. RAS-N031) and P.1 RBD (Cat. No. RAS-N034) are used to detect neutralizing antibodies in 56 post-vaccination serum samples in order to evaluate vaccine efficacy against SARS-CoV-2 Variants of Concerns (VOC).
| Lineage | Cat. No. | Product Description | Preorder/Order |
|---|
>>>Click to find out more ELISA kits for IgM/ Total antibody detection
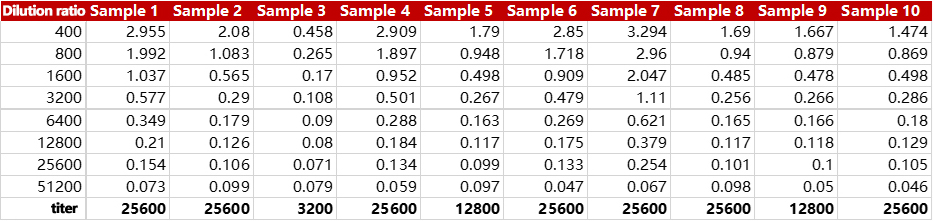
Cat. No. RAS-T024 can accurately test the antibody titer in vaccinated serum. The batch-to-batch difference and precision were all less than 10%.
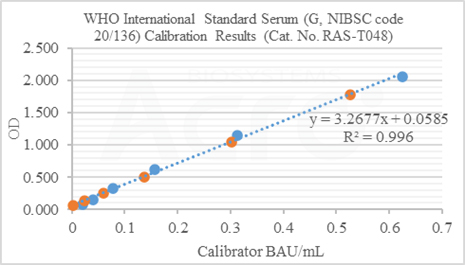
Anti-SARS-CoV-2 Antibody IgG Quantitative Detection Kit (Spike Trimer) (Cat. No. RAS-T048) is developed for quantitative detection of IgG antibodies in post-vaccination serum. This kit is an important supplement to the existing vaccine evaluation methods which significantly improve the efficiency and accuracy of vaccine evaluation.
| Lineage | Cat. No. | Product Description | Preorder/Order |
|---|
>>>Click to find out more ELISA kits for IgM/ Total antibody detection
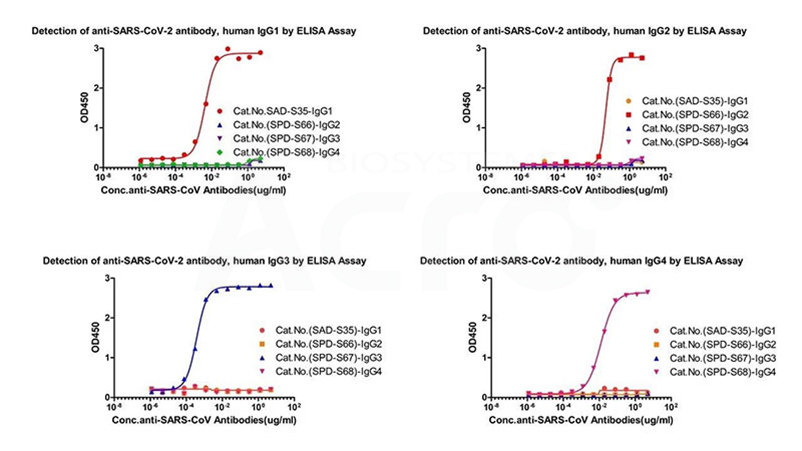
cross-validation data for antibody subtype detection
>>> If you have any customized inquiries or suggestions for SARS-CoV-2 (COVID-19) related product development, please click here.
This web search service is supported by Google Inc.