Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Antibody-drug conjugates (ADCs), an emerging class of targeted therapies that witnessed substantial growth over the past few years, are gradually starting the revolution of clinical cancer therapy.
You will learn the following key information for ADC drug development after reading the text:
• Specificity and expression of target
• Affinity and internalization of antibody
• Mechanism and properties of payload
• Covalent and labile linkers
• Conjugation chemistry
In recent years, ADCs may serve as a novel therapeutic modality for many cancer patients. ADCs are a kind of modified antibody drug that consists of three parts: monoclonal antibody (mAb), linker, and cytotoxin/payload. The monoclonal antibody binds to specific antigens on the surface of target tumor cells and brings the payload for desired effect. Compared with traditional chemical and biological drugs, the payload delivered to the tumor directly is much potent and reduced off-target toxicities. However, many factors can affect the efficiency of ADCs including specific target, suitable antibody and payload, stable and efficient linkers.
Key point of ADCs design
The target/antigen is the starting point in developing ADCs and determines which tumor indication will be targeted by ADCs and potentially impacts the choice of the conjugated drug payload. Choosing the right target is critical for ADCs development. The target will be related to drug recognized, special binding and drug’s effect. Widely used target are cluster of differentiations such as CD22, CD30, CD79B, CD123 etc. These targets are prevalent in hematological malignancies like leukemia and lymphoma. For solid tumors, targets such as Her2 / Nectin-4 / TROP-2 / PSMA are being researched currently. There are four key points when selecting the suitable ADCs format.
Specificity
· The core principle of ADCs approach
· Specific expression or high expression of antigen in tumor and cancer cells
· No or low expression in normal cells
Express abundance
· The target expression level is high enough on the cell surface to guarantee the effectiveness of ADCs
Internalization
· ADCs can enter the lysosomal pathway and be recycled quickly. An increased rate of internalization could potentially result in increased intracellular delivery of the cytotoxic agent
Antigen expression variability
· Varying expression level in different parts of cancer/tumor tissue within the same period
· Varying expression level in the same cancer/tumor tissue at different stages
· Individual patient variability
The high specificity of antibody is the basic requirement for the efficacy of ADCs. The payload will enter the tumor relying on these high-affinity specific antibodies. In addition to avoiding non-specific binding, the payloads are usually conjugated to the Fc fragment or constant region of the mAb to enable cellular internalization. Majority of ADCs are based on IgG1 antibodies containing Fc fragment which will induce immune functions such as ADCC, CDC, and ADCP. Another consideration affecting cellular internalization is ADCs antibody size which limits the internalization rate. High affinity binding with FcRn can cause ADCs to return to the extracellular circulation, thus increasing exposure to healthy tissues which not only limits the release of intracellular payloads but may also cause off-target toxicities. FcγR cross-reacts with endothelial cells and the immune system, and may also cause off-target toxicity. There are four points when choice the suitable proposal as follow:
Immunogenicity
· Mouse source → Human source
Isotype of the antibody
· ADCs currently in oncology clinical trials are canonical (i.e., full length) IgG molecules, mostly IgG1 isotype, because IgG1 mAbs have the potential to mediate CDC.
Cross reaction
· Binding antigen among different species
Internalization
· Fast & effective formation of lysosomes
Half life
· 19-23 days (additional modification may reduce half-life)
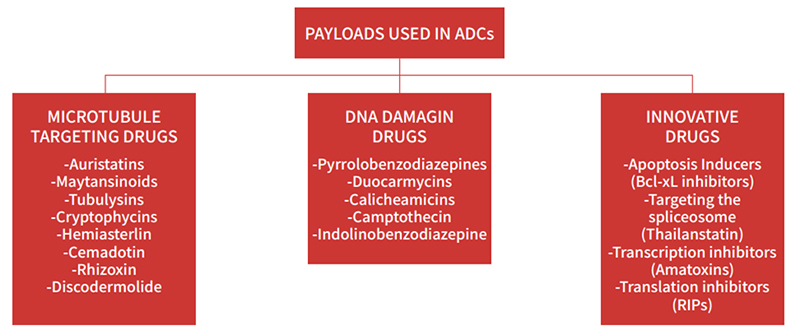
The first ADCs utilized readily available conventional cytotoxins such as doxorubicin, methotrexate, mitomycin, fluorouracil and vinca alkaloid. These chemotherapeutic drugs have relatively low potency with half maximal inhibitory concentration (IC50) values in the micromolar range. However, they lacked selectivity and their accumulation in target cells was poor. As a result, early ADCs had relatively low efficacy. Currently, most ADCs are constructed with two main families of highly toxic compounds acting either on microtubule or the DNA structure. The most commonly used DNA targeting payloads include calicheamicins, duocarmycins, pyrrolobenzodiazepines (PBDs), SN-38 and DXd. Additionally, the anti-tubulin agents include auristatins and maytansinoids etc.
Linkers play a key role in ADCs design. Various factors such as linker chemistry, mode and the site of conjugation play a critical role in the PK/PD profiles of ADCs. Linkers must maintain stability of ADCs in the blood long enough to reach the cancer cell but must then be readily cleaved when internalized so that the payload can be released. Broadly speaking, linkers can be categorized into two groups: cleavable or non-cleavable linkers. Cleavable linkers depend on physiological conditions in the cell to cleave the linker and can be further subdivided into acid labile, protease sensitive and glutathione sensitive. Non-cleavable linkers on the other hand form non-reducible bonds with the amino acid residues of the mAb and are therefore more stable in the bloodstream, have longer half-lives and reduced off target toxicity.
The conjugation chemistry between antibody and the payload can alter the PK and therapeutic index of ADCs. The conventional drug conjugation usually occurs on the mAb backbone either via alkylation or acylation of lysine sidechains. These drugs (i.e gemtuzumab ozogamicin, trastuzumab emtansine) conjugation strategies are random and can produce a heterogeneous mixture of ADCs with a varying number of Drug Antibody Ratio (DAR). The DAR can range anywhere from zero to eight payloads per antibody. Although high DAR can produce more potent ADCs, it can also result in destabilization, aggregation, increased off-target toxicity, and enhanced drug clearance from systemic circulation. Hence, there is more attention in site-specific conjugation (SSC) that can produce more homogenous ADCs. These strategies include insertion of engineered cysteine residues, insertion of unnatural amino acids in the antibody sequence or enzymatic conjugation through glycotransferases and transglutaminases. These ADCs with site-specific conjugation are in different clinical phase.
ADCs are drugs with enormous potential for the treatment of hematological malignancies and solid tumors. Strategies to manage toxicities and improve efficacy warrant further exploration. The design of ADCs requires comprehensive consideration of various factors to ensure their specificity, efficacy and stability.
References
[1]Parslow, Adam, C, et al. Antibody–Drug Conjugates for Cancer Therapy[J]. Biomedicines, 2016.
[2] Ponziani S , Vittorio G D , Pitari G , et al. Antibody-Drug Conjugates: The New Frontier of Chemotherapy[J]. International Journal of Molecular Sciences, 2020, 21(15):5510.
[3] Denevault-Sabourin C , Joubert N , Beck A , et al. Antibody- Drug Conjugates: The Last Decade[J]. Pharmaceuticals, 2020, 13(9):245.
This web search service is supported by Google Inc.