 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| SCCHO-ATP171 | Human | CHO/Human CD79B Stable Cell Line Development Service | |||
| CDB-HP2H6 | Human | PE-Labeled Human CD79B Protein, His Tag (Site-specific conjugation) |  |
||
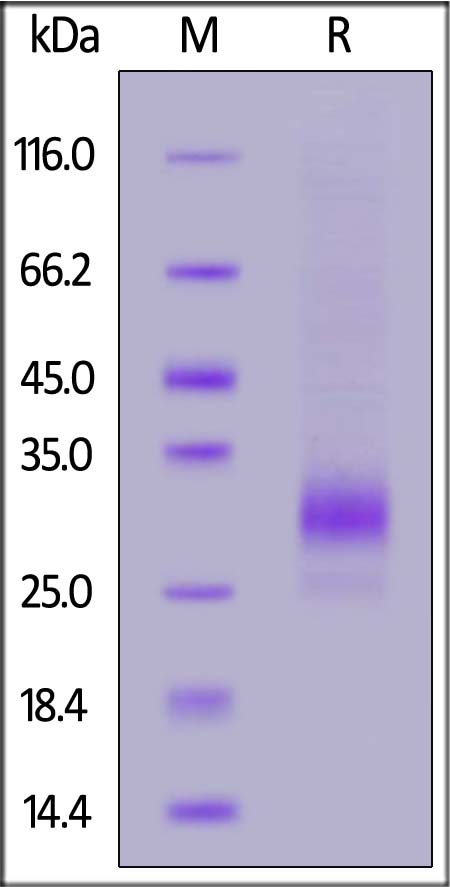
| CDB-C82E5 | Cynomolgus | Biotinylated Cynomolgus CD79B Protein, His,Avitag™ |  |
|
|
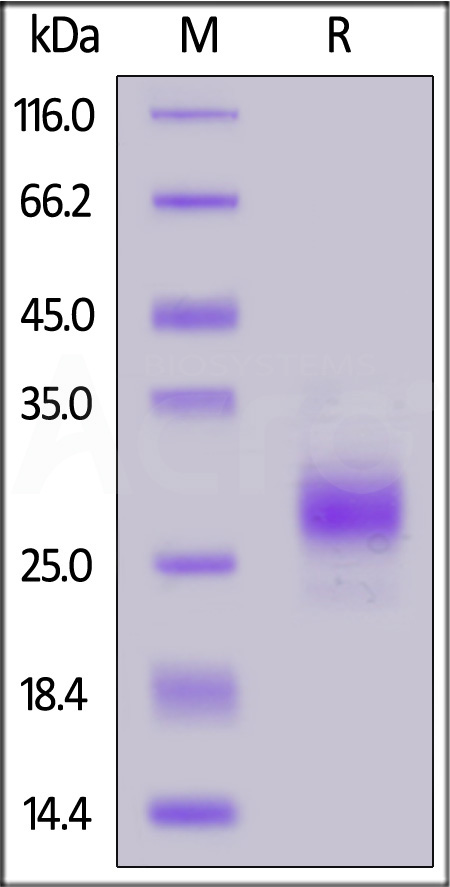
| CDB-C52H3 | Cynomolgus | Cynomolgus CD79B Protein, His Tag (MALS verified) |  |
|
|
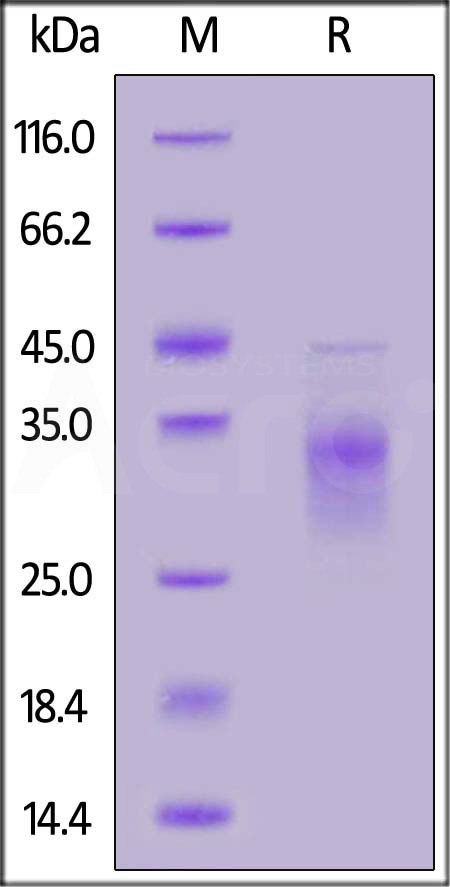
| CDB-H52H3 | Human | Human CD79B Protein, His Tag (MALS verified) |  |
|
|
| CDB-H82E3 | Human | Biotinylated Human CD79B Protein, His,Avitag™ (MALS verified) |  |
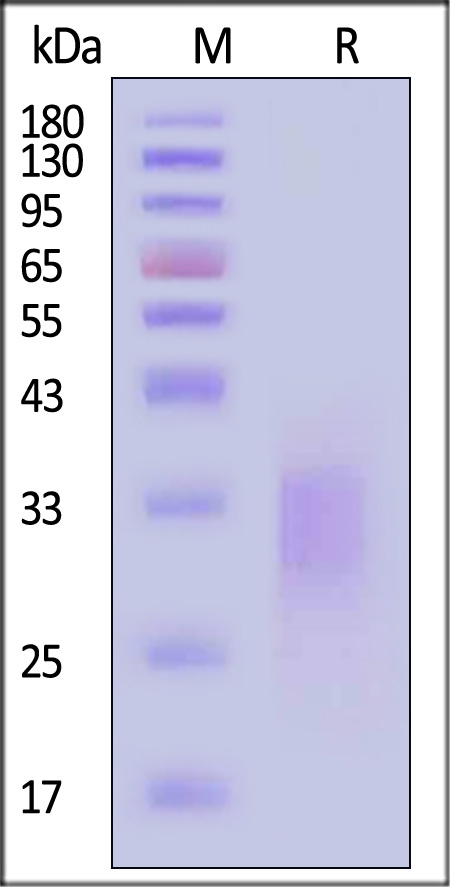
|
|
| CDB-M52H3 | Mouse | Mouse CD79B Protein, His Tag (MALS verified) |  |
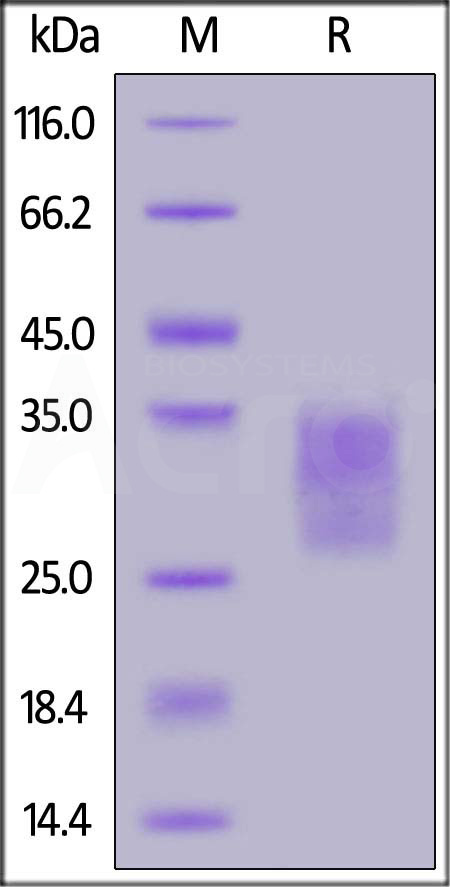
|
|
| CDB-M5253 | Mouse | Mouse CD79B Protein, Fc Tag (MALS verified) |  |
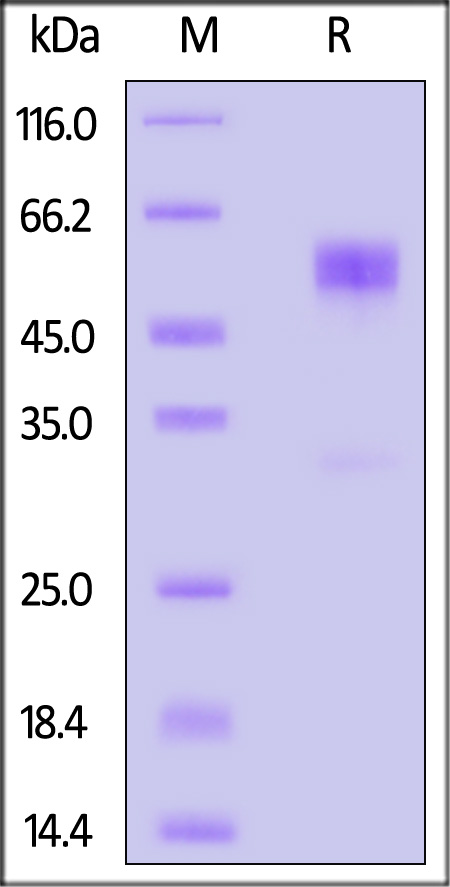
|
|
| CDB-M52H4 | Mouse | Mouse CD79B Protein, His Tag, low endotoxin (MALS verified) |  |
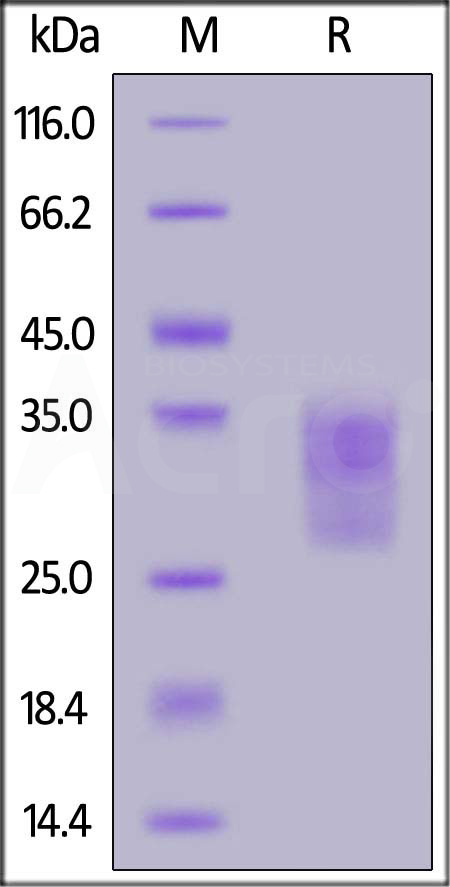
|
|
| CDB-H5259 | Human | Human CD79B Protein, Fc Tag |  |

|
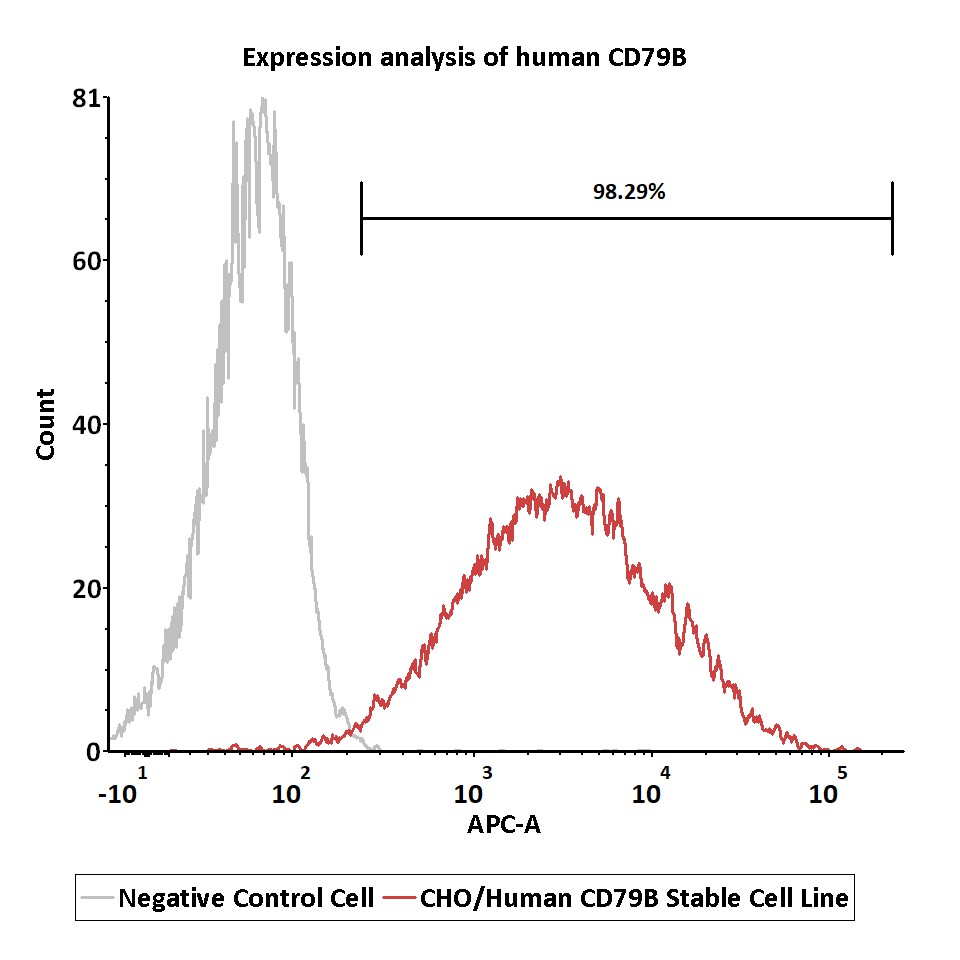
Expression analysis of human CD79B on CHO/Human CD79B Stable Cell Line by FACS.
Cell surface staining was performed on CHO/Human CD79B Stable Cell Line or negative control cell using APC-labeled anti-human CD79B antibody.
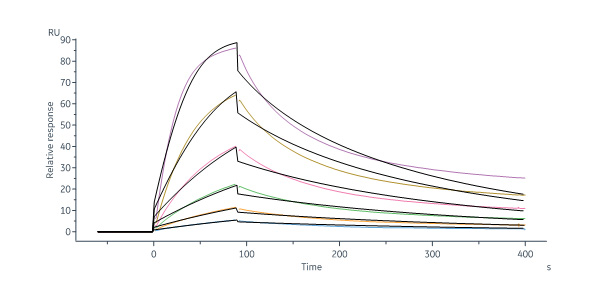
Polatuzumab captured on Protein G Chip can bind Human CD79B, His Tag (Cat. No. CDB-H52H3) with an affinity constant of 1.97 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Polatuzumab vedotin | RO-5541077-000; FCU-2711; DCDS-4501A; RG-7596; RO-5541077 | Approved | Genentech Inc | Polivy | United States | Lymphoma, Large B-Cell, Diffuse | Genentech Inc | 2019-06-10 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Polatuzumab vedotin | RO-5541077-000; FCU-2711; DCDS-4501A; RG-7596; RO-5541077 | Approved | Genentech Inc | Polivy | United States | Lymphoma, Large B-Cell, Diffuse | Genentech Inc | 2019-06-10 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| PRV-3279 | HDM-3002; MGD-010; PRV-3279; CD32BxCD79B | Phase 2 Clinical | Macrogenics Inc | Lupus Erythematosus, Systemic | Details |
| CD19/79b Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR CD19/79b | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| TolDCB29 | Phase 2 Clinical | Umc Utrecht | Arthritis, Rheumatoid | Details | |
| SHR-A1912 | SHR-A1912 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| CD79b-19 CAR T Cells Therapy (Massachusetts General Hospital) | Phase 1 Clinical | Massachusetts General Hospital | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details | |
| Autologous CD79b-targeting Chimeric Antigen Receptor T-cell Therapy(MD Anderson Cancer Center) | JV-213 | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, B-Cell; Lymphoma | Details |
| NBT-508 | Phase 1 Clinical | Newbio Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD79b CAR-T cell therapy (Yake Biotechnology) | Phase 1 Clinical | Zhejiang University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Biphenotypic, Acute | Details | |
| Iladatuzumab vedotin | DCDS-0780A; RO-7032005 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Lymphoma, Non-Hodgkin | Details |
| PRV-3279 | HDM-3002; MGD-010; PRV-3279; CD32BxCD79B | Phase 2 Clinical | Macrogenics Inc | Lupus Erythematosus, Systemic | Details |
| CD19/79b Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR CD19/79b | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| TolDCB29 | Phase 2 Clinical | Umc Utrecht | Arthritis, Rheumatoid | Details | |
| SHR-A1912 | SHR-A1912 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| CD79b-19 CAR T Cells Therapy (Massachusetts General Hospital) | Phase 1 Clinical | Massachusetts General Hospital | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details | |
| Autologous CD79b-targeting Chimeric Antigen Receptor T-cell Therapy(MD Anderson Cancer Center) | JV-213 | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, B-Cell; Lymphoma | Details |
| NBT-508 | Phase 1 Clinical | Newbio Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD79b CAR-T cell therapy (Yake Biotechnology) | Phase 1 Clinical | Zhejiang University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Biphenotypic, Acute | Details | |
| Iladatuzumab vedotin | DCDS-0780A; RO-7032005 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Lymphoma, Non-Hodgkin | Details |
This web search service is supported by Google Inc.
