 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
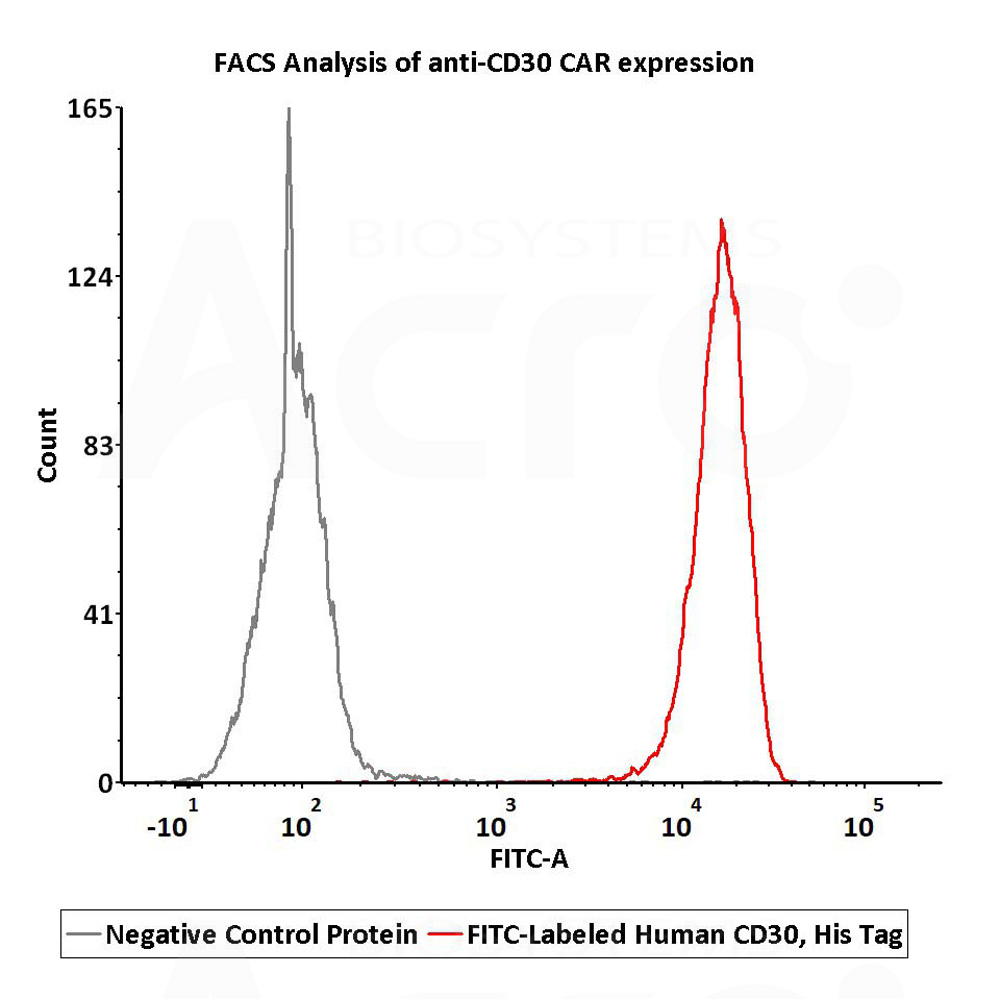
2e5 of anti-CD30 CAR-293 cells were stained with 100 μL of 1 μg/mL of FITC-Labeled Human CD30, His Tag (Cat. No.CD0-HF2H4) and negative control protein respectively, FITC signal was used to evaluate the binding activity (QC tested).
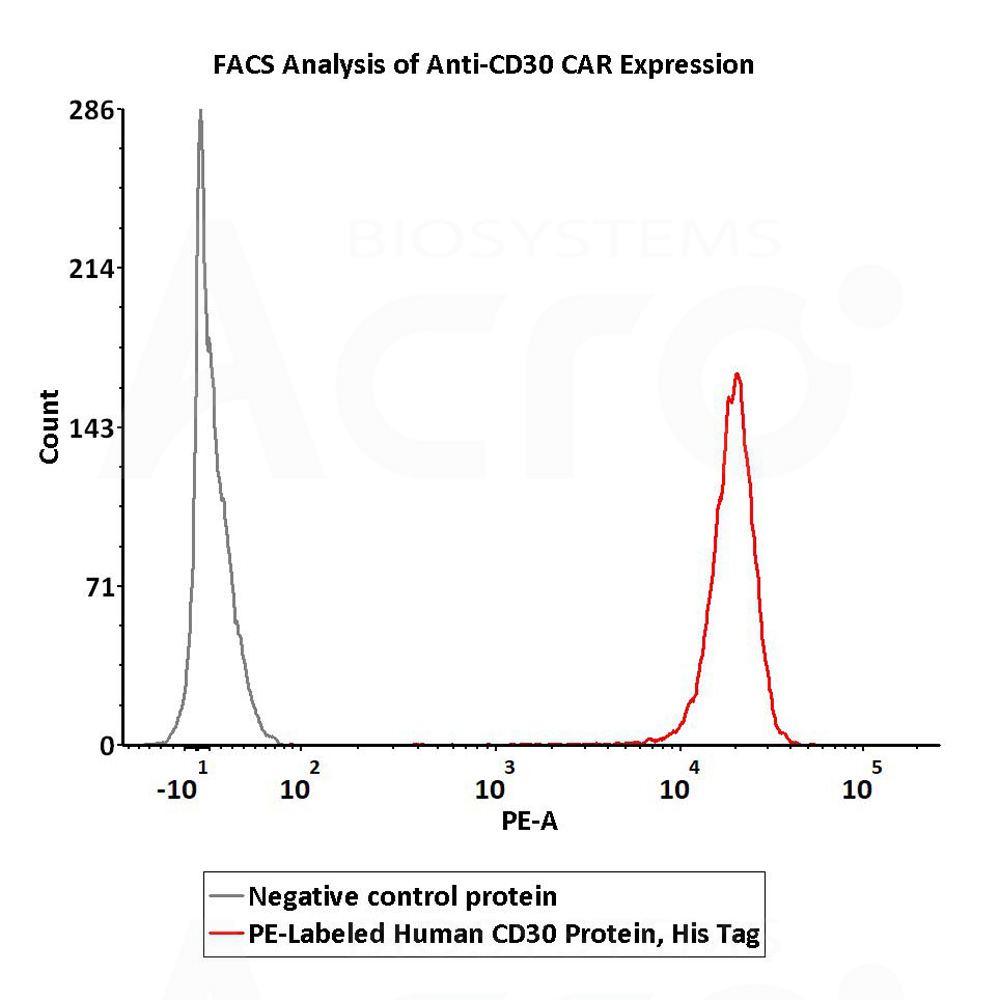
5e5 of anti-CD30 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD30, His Tag (Cat. No. CD0-HP2E3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
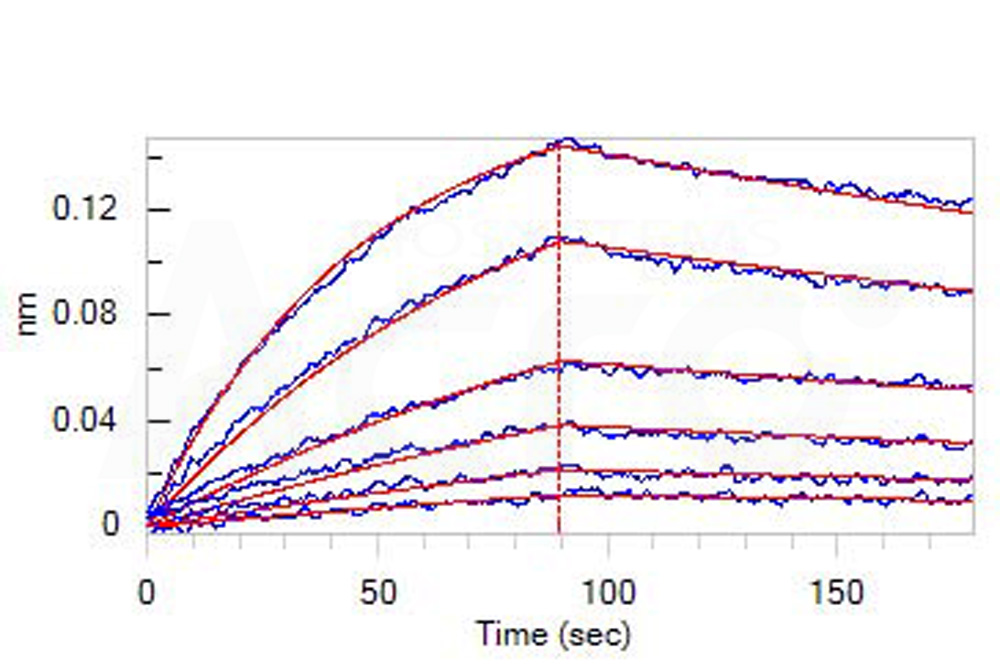
Loaded Human CD30 Ligand, His Tag (Cat. No. CDL-H524b) on HIS1K Biosensor, can bind Human CD30 Protein, Llama IgG2b Fc Tag (Cat. No. TN8-H5250) with an affinity constant of 55.5 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Brentuximab vedotin | SGN-30; SGN-35; cAC-10; SGD-1010; cAC10-vcMMAE; cAC10-Val-Cit-MMAE | Approved | Millennium Pharmaceuticals Inc | Adcetris, 安适利 | United States | Lymphoma, Large-Cell, Anaplastic; Hodgkin Disease | Seagen Inc | 2011-08-19 | Lymphoma, T-Cell; Lymphoma, Primary Cutaneous Anaplastic Large Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Sezary Syndrome; Scleroderma, Diffuse; Lymphoma, T-Cell, Cutaneous; Lymphoma, Large-Cell, Anaplastic; Leukemia, Myeloid, Acute; Leukemia, Mast-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Hidradenitis Suppurativa; Mycosis Fungoides; Lymphomatoid Papulosis; Sarcoma, Kaposi; Neoplasms, Germ Cell and Embryonal; Lymphoma, T-Cell, Peripheral; Mesothelioma; Lymphoma, Large B-Cell, Diffuse; Enteropathy-Associated T-Cell Lymphoma; Neoplasms; Myelodysplastic Syndromes; Mastocytosis, Systemic; Hodgkin Disease; Scleroderma, Systemic; Graft vs Host Disease; Carcinoma; Anemia, Refractory, with Excess of Blasts; HIV Infections; Lymphoma, B-Cell; Solid tumours; Hematologic Diseases | Details |
| Brentuximab vedotin | SGN-30; SGN-35; cAC-10; SGD-1010; cAC10-vcMMAE; cAC10-Val-Cit-MMAE | Approved | Millennium Pharmaceuticals Inc | Adcetris, 安适利 | United States | Lymphoma, Large-Cell, Anaplastic; Hodgkin Disease | Seagen Inc | 2011-08-19 | Lymphoma, T-Cell; Lymphoma, Primary Cutaneous Anaplastic Large Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Sezary Syndrome; Scleroderma, Diffuse; Lymphoma, T-Cell, Cutaneous; Lymphoma, Large-Cell, Anaplastic; Leukemia, Myeloid, Acute; Leukemia, Mast-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Hidradenitis Suppurativa; Mycosis Fungoides; Lymphomatoid Papulosis; Sarcoma, Kaposi; Neoplasms, Germ Cell and Embryonal; Lymphoma, T-Cell, Peripheral; Mesothelioma; Lymphoma, Large B-Cell, Diffuse; Enteropathy-Associated T-Cell Lymphoma; Neoplasms; Myelodysplastic Syndromes; Mastocytosis, Systemic; Hodgkin Disease; Scleroderma, Systemic; Graft vs Host Disease; Carcinoma; Anemia, Refractory, with Excess of Blasts; HIV Infections; Lymphoma, B-Cell; Solid tumours; Hematologic Diseases | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Acimtamig | AFM-13 | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin; Mycosis Fungoides | Details |
| ATLCAR.CD30 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 2 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, T-Cell, Peripheral; Lymphatic Diseases; Immunoproliferative Disorders; Hodgkin Disease; Neoplasms; Immune System Diseases; Lymphoproliferative Disorders; Lymphoma, Non-Hodgkin; Lymphoma; Neoplasms, Germ Cell and Embryonal | Details | |
| Itezocabtagene autoleucel | TT-11; TT11 | Phase 2 Clinical | Tessa Therapeutics Ltd | Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic | Details |
| BRD-01 | BRD-01 | Phase 2 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Hematologic Neoplasms; Hodgkin Disease | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details | |
| HSP-CAR-30 | HSP-CAR-30 | Phase 2 Clinical | Fundació Institut De Recerca De L | Hodgkin Disease; Lymphoma, T-Cell | Details |
| Anti-CD30 CAR T-cell therapy (General Hospital of the People's Liberation Army/Cellular Biomedicine) | CAR30-NKT; CBM-C30.1; CD30ScFv-CD8-CD137-CD3zeta | Phase 2 Clinical | Pla General Hospital | Hodgkin Disease | Details |
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Pancreatic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD30 chimeric antigen receptor T cell therapy (Immune cell) | Phase 1 Clinical | Immune Cell Inc | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details | |
| CD30.CAR-EBVST cell therapy (Baylor College of Medicine) | TT-11X | Phase 1 Clinical | Baylor College Of Medicine, Tessa Therapeutics Ltd | Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell | Details |
| EBV-specific-CAR.CD30 | EBV-specific-CAR.CD30; CAR.CD30 EBV-specific-CTLs | Phase 1 Clinical | Baylor College Of Medicine, Texas Children'S Hospital, Methodist Hospital System | Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| Anti-CD30 CAR T-cell therapy (Wuhan Bio-Raid) | Phase 1 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Hematologic Neoplasms; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Leukemia-Lymphoma, Adult T-Cell; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell | Details | |
| Recombinant chimeric anti-CD30 monoclonal antibody-MCC-DM1 | F0002-ADC; B-006 | Phase 1 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd, Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Lymphoma, T-Cell, Peripheral; Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details |
| CD30.CAR | H-27721; CD30.CAR | Phase 1 Clinical | Baylor College Of Medicine | Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Hodgkin Disease; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic | Details |
| SGN-35C | SGN-35C | Phase 1 Clinical | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Large-Cell, Anaplastic; Lymphoma | Details | |
| SGN-35T | SGN-CD30C; PF-08046045; SGN-35T | Phase 1 Clinical | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, T-Cell, Cutaneous; Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic | Details | |
| Anti-CD30 CAR-T cell therapy (National Cancer Institute) | Hu30-CD28z | Phase 1 Clinical | National Cancer Institute | Lymphoma, T-Cell, Peripheral; Enteropathy-Associated T-Cell Lymphoma; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma | Details |
| PRA-052 | PRA-052 | Phase 1 Clinical | Prometheus Biosciences Inc | Colitis, Ulcerative | Details |
| Anti CD30 CAR T Cell Therapy (First Song Therapeutics) | Phase 1 Clinical | Zhejiang University | Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Lymphoma, Extranodal NK-T-Cell; Lymphoma; Lymphoma, T-Cell; Lymphoma, Large-Cell, Anaplastic | Details | |
| SGN-CD30C | SGN-CD30C | Phase 1 Clinical | Seattle Genetics Inc | Lymphoma | Details |
| Acimtamig | AFM-13 | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin; Mycosis Fungoides | Details |
| ATLCAR.CD30 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 2 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, T-Cell, Peripheral; Lymphatic Diseases; Immunoproliferative Disorders; Hodgkin Disease; Neoplasms; Immune System Diseases; Lymphoproliferative Disorders; Lymphoma, Non-Hodgkin; Lymphoma; Neoplasms, Germ Cell and Embryonal | Details | |
| Itezocabtagene autoleucel | TT-11; TT11 | Phase 2 Clinical | Tessa Therapeutics Ltd | Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic | Details |
| BRD-01 | BRD-01 | Phase 2 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Hematologic Neoplasms; Hodgkin Disease | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details | |
| HSP-CAR-30 | HSP-CAR-30 | Phase 2 Clinical | Fundació Institut De Recerca De L | Hodgkin Disease; Lymphoma, T-Cell | Details |
| Anti-CD30 CAR T-cell therapy (General Hospital of the People's Liberation Army/Cellular Biomedicine) | CAR30-NKT; CBM-C30.1; CD30ScFv-CD8-CD137-CD3zeta | Phase 2 Clinical | Pla General Hospital | Hodgkin Disease | Details |
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Pancreatic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD30 chimeric antigen receptor T cell therapy (Immune cell) | Phase 1 Clinical | Immune Cell Inc | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details | |
| CD30.CAR-EBVST cell therapy (Baylor College of Medicine) | TT-11X | Phase 1 Clinical | Baylor College Of Medicine, Tessa Therapeutics Ltd | Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell | Details |
| EBV-specific-CAR.CD30 | EBV-specific-CAR.CD30; CAR.CD30 EBV-specific-CTLs | Phase 1 Clinical | Baylor College Of Medicine, Texas Children'S Hospital, Methodist Hospital System | Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| Anti-CD30 CAR T-cell therapy (Wuhan Bio-Raid) | Phase 1 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Hematologic Neoplasms; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Leukemia-Lymphoma, Adult T-Cell; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell | Details | |
| Recombinant chimeric anti-CD30 monoclonal antibody-MCC-DM1 | F0002-ADC; B-006 | Phase 1 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd, Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Lymphoma, T-Cell, Peripheral; Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details |
| CD30.CAR | H-27721; CD30.CAR | Phase 1 Clinical | Baylor College Of Medicine | Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Hodgkin Disease; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic | Details |
| SGN-35C | SGN-35C | Phase 1 Clinical | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Large-Cell, Anaplastic; Lymphoma | Details | |
| SGN-35T | SGN-CD30C; PF-08046045; SGN-35T | Phase 1 Clinical | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, T-Cell, Cutaneous; Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic | Details | |
| Anti-CD30 CAR-T cell therapy (National Cancer Institute) | Hu30-CD28z | Phase 1 Clinical | National Cancer Institute | Lymphoma, T-Cell, Peripheral; Enteropathy-Associated T-Cell Lymphoma; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma | Details |
| PRA-052 | PRA-052 | Phase 1 Clinical | Prometheus Biosciences Inc | Colitis, Ulcerative | Details |
| Anti CD30 CAR T Cell Therapy (First Song Therapeutics) | Phase 1 Clinical | Zhejiang University | Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Lymphoma, Extranodal NK-T-Cell; Lymphoma; Lymphoma, T-Cell; Lymphoma, Large-Cell, Anaplastic | Details | |
| SGN-CD30C | SGN-CD30C | Phase 1 Clinical | Seattle Genetics Inc | Lymphoma | Details |
This web search service is supported by Google Inc.
