 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> IgG Fc Proteins——Ideal Isotype Control for IgG Structured Drug Development

Immunoglobulin G (IgG) antibodies have attracted great attention in the pharmaceutical industry due to its high specificity and superior pharmacokinetics since the late 1990s, especially following the inaugural approval of Ritux*mab, the first anti-tumor antibody drug, in 1997. Nowadays, IgG antibodies have been used in the treatment of cancer and autoimmune diseases with great success. Fc-mediated effector functions (ADCC, ADCP, CDC) play an important role in the therapeutic effects among the mechanisms by which various IgG antibody drugs exert their therapeutic effects.
There are four distinct subclasses of human IgG with decreasing concentrations, namely IgG1, IgG2, IgG3, and IgG4 (approximately 61%,32%,4%,3%). Although all subclasses are more than 90% identical at the amino acid level and have similar spatial structures. They are highly conserved in terms of the length of the hinge regions, the number of disulfide bonds, and the Fc effect function. These regions are involved in binding to both Ig-FC receptors (FcγR) and C1q. As a result, the different subclasses have different effector functions, both in terms of triggering FcγR-expressing cells, resulting in phagocytosis (ADCP) or antibody-dependent cell mediated cytotoxicity (ADCC), and activating complement (CDC).
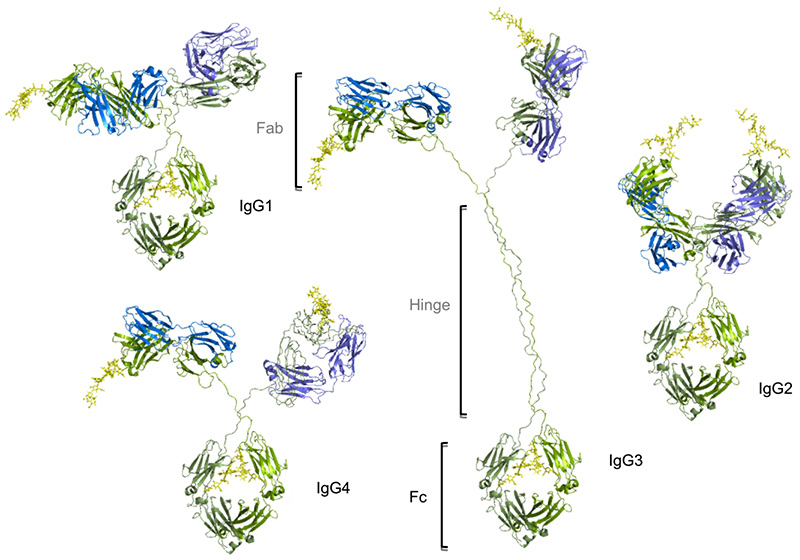
IgG1 is the most potential subclass in tumor immunotherapy. In addition to Human IgG1 can also effectively bind Mouse Fcγs, significant effects can also be observed in vivo models. The half-life of IgG1 in the body is relatively long. Moreover, from an industrial perspective, IgG1 can be highly expressed and can be purified by protein-A, which makes it highly economical and stable. Therefore, IgG1 has become the most commonly used IgG subtype.
IgG2 is mainly used to neutralize the antigen or block the binding of receptor ligands. The ADCC effect is very weak. Although the binding to C1q is relatively weak, but it can still trigger the CDC effect when the antigen or antibody solubility is high. At the same time, IgG2 is the only subtype that can bind to FcγRIIa (CD32a). In the early days, only antibodies targeting EGFR were marketed based on the IgG2 subtype. With the rise of immune checkpoint research, more and more drugs of the IgG2 subtype have entered the clinic and are on the market.
![]() Expressed by HEK293 cells: which realize post-translational glycosylation and other modifications and correct protein folding
Expressed by HEK293 cells: which realize post-translational glycosylation and other modifications and correct protein folding
Human IgG1 Fc, IgG2 Fc, IgG3 Fc, IgG4 Fc | |
Mouse IgG1 Fc, IgG2a Fc, IgG2b Fc | |
Llama IgG2b Fc,Rabbit IgG Fc |
![]() Various tags: Tag Free,AvitagTM,His Tag,gD Tag,Flag Tag,AvitagTM &His tag
Various tags: Tag Free,AvitagTM,His Tag,gD Tag,Flag Tag,AvitagTM &His tag
![]() Low endotoxin: Less than 1.0 EU/μg by the LAL method
Low endotoxin: Less than 1.0 EU/μg by the LAL method
more than 95% as verified by SDS-PAGE | |
more than 90% as verified by SEC-MALS |
![]() High bioactivity: verified by ELISA & SPR with free protocols
High bioactivity: verified by ELISA & SPR with free protocols
| Molecule | Cat. No. | Species | Product Description | Preorder/Order |
|---|---|---|---|---|
| IgG1 Fc | FCC-H5214 | Human | Human IgG1 Fc Protein, Tag Free (MALS verified) | |
| IgG1 Fc | IG1-H8213 | Human | Biotinylated Human IgG1 Fc protein, Avitag™ (MALS verified) | |
| IgG1 Fc | IG1-H5225 | Human | Human IgG1 Fc Protein, His Tag (MALS verified) | |
| IgG1 Fc | IG1-H52C9 | Human | Human IgG1 Fc Protein, Flag Tag (MALS verified) | |
| IgG1 Fc | IG1-H52G6 | Human | Human IgG1 Fc Protein, gD Tag (MALS verified) | |
| IgG2 Fc | IG2-H5206 | Human | Human IgG2 Fc Protein, Tag Free (MALS & SPR verified) | |
| IgG3 Fc | IG3-H5200 | Human | Human IgG3 Fc Protein, Tag Free (MALS & SPR verified) | |
| IgG4 Fc | IG4-H5205 | Human | Human IgG4 Fc Protein, Tag Free (MALS & SPR verified) | |
| IgG1 Fc | IG1-M5208 | Mouse | Mouse IgG1 Fc Protein, Tag Free (HPLC verified) | |
| IgG1 Fc | IG1-M8211 | Mouse | Biotinylated Mouse IgG1 Fc protein, Avitag™ (MALS verified) | |
| IgG2a Fc | IGA-M5207 | Mouse | Mouse IgG2a Fc Protein, Tag Free (MALS verified) | |
| IgG2a Fc | IGA-M8210 | Mouse | Biotinylated Mouse IgG2a Fc Protein, Avitag™ (MALS verified) | |
| IgG2b Fc | IGB-M5203 | Mouse | Mouse IgG2b Fc Protein, Tag Free (MALS verified) | |
| IgG2b Fc | IGB-L5204 | Llama | Llama IgG2b Fc Protein, Tag Free (MALS verified) | |
| IgG Fc | IGG-R5203 | Rabbit | Rabbit IgG Fc Protein, Tag Free (MALS verified) |
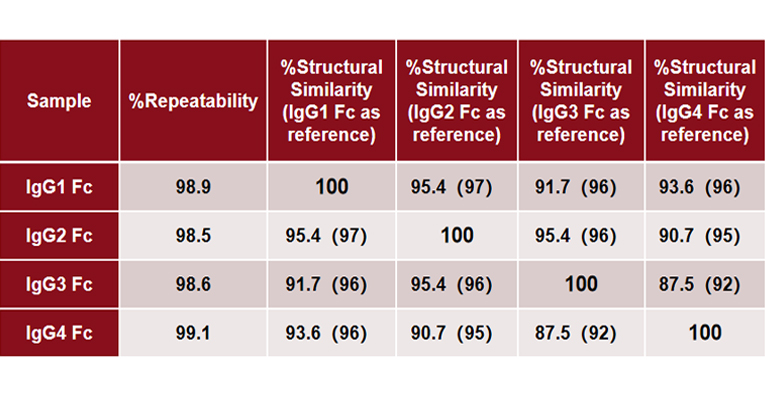
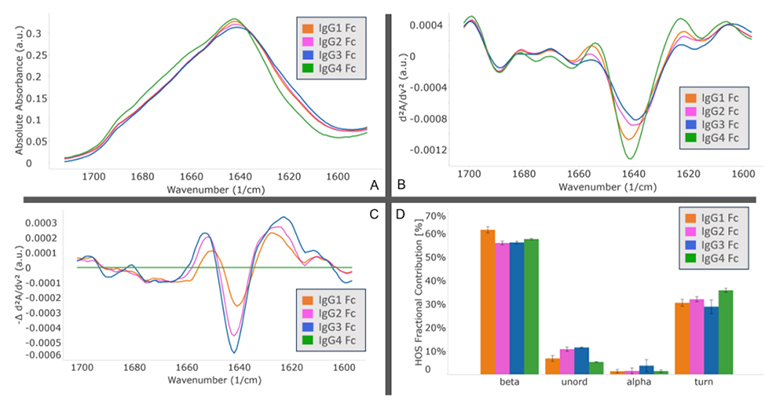
MMS analysis showed that different IgG Fc subclasses exhibit distinct secondary structures. Specifically, IgG1 and IgG2 exhibit the highest degree of structural similarity, while IgG3 and IgG4 show the greatest structural difference.
Explore the In-depth Secondary Structure Analysis of IgG Fc
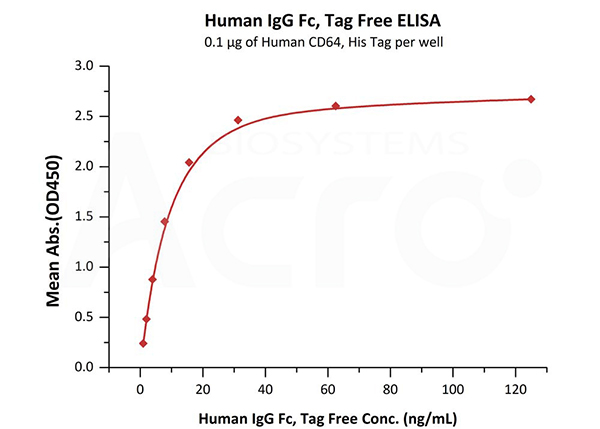
Immobilized Human CD64, His Tag (Cat. No. FCA-H52H1) at 1 μg/mL (100 μL/well) can bind Human IgG Fc, Tag Free (Cat. No. FCC-H5214) with a linear range of 1-16 ng/mL.
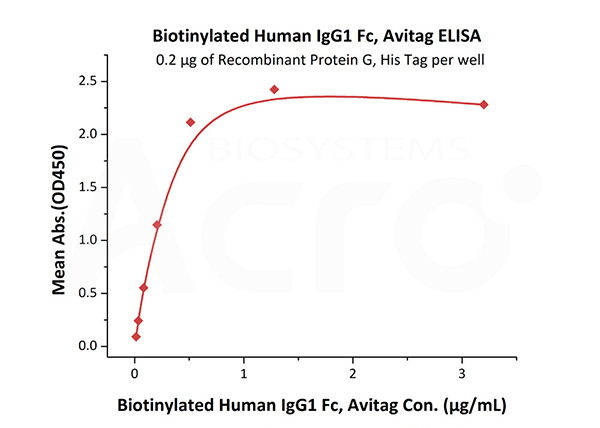
Immobilized Recombinant Protein G, His Tag (Cat. No. RPG-S3140) at 2 μg/mL (100 μL/well) can bind Biotinylated Human IgG1 Fc, Avitag (Cat. No. IG1-H8213) with a linear range of 0.013-0.512 μg/mL.
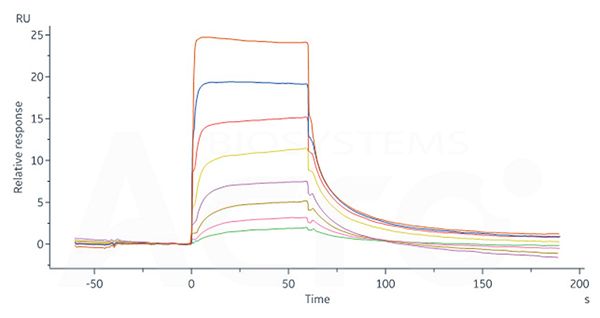
Human FCGRT&B2M Heterodimer Protein, His Tag (Cat. No. FCN-H52W7) captured on CM5 Chip via anti-His antibody can bind Human IgG1 Fc, Tag Free (Cat. No. FCC-H5214) with an affinity constant of 0.957 μM as determined in SPR assay (Biacore 8K).
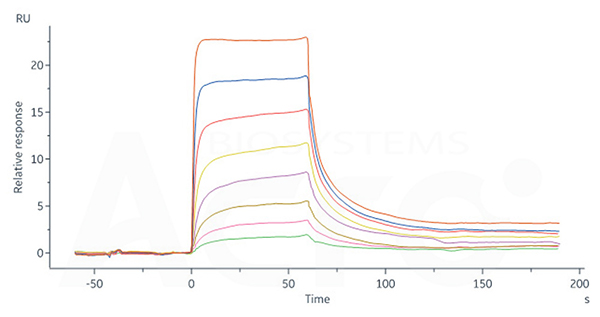
Human FCGRT&B2M Heterodimer Protein, His Tag (Cat. No. FCN-H52W7) captured on CM5 Chip via anti-His antibody can bind Human IgG4 Fc, Tag Free (Cat. No. IG4-H5205) with an affinity constant of 0.715 μM as determined in SPR assay (Biacore 8K).
Mouse IgG2a Fc protein is used as an isotype control to verify the specific binding of Mouse IgG2a CD3 monoclonal antibody (Clone: OKT3) to Human CD3 protein.
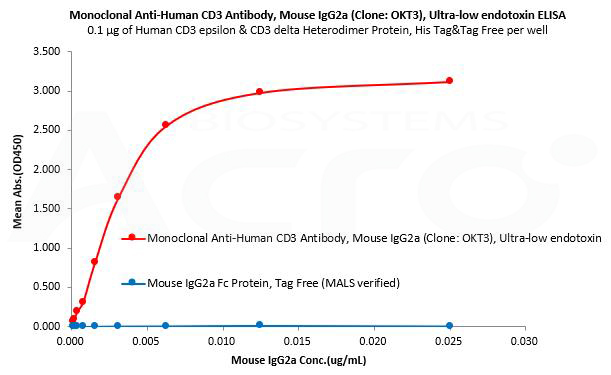
Human CD3 epsilon & CD3 delta Heterodimer Protein, His Tag&Tag Free (MALS verified) (Cat. No. CDD-H52W1) can bind Monoclonal Anti-Human CD3 Antibody, Mouse IgG2a (Clone: OKT3), Ultra-low endotoxin (Cat. No. CDE-M120a) with a linear range of 0.2-3 ng/mL and cannot bind Mouse IgG2a Fc Protein, Tag Free (MALS verified) (Cat. No. IGA-M5207).
Human IgG1 Fc is used as an isotype control to verify the specific binding of anti-BCMA×CD3 scFv- Human IgG1 Fc Tag to Human CD3 protein.
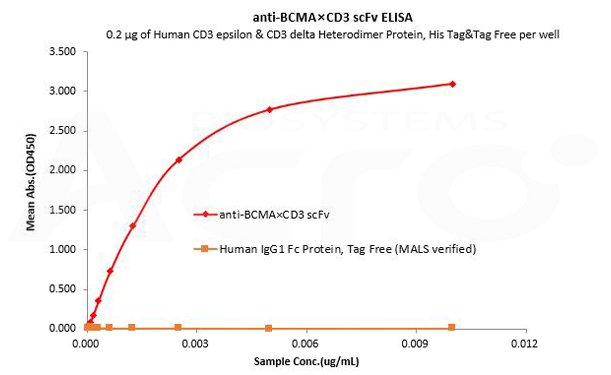
Human CD3 epsilon & CD3 delta Heterodimer Protein, His Tag&Tag Free (MALS verified) (Cat. No. CDD-H52W1) can bind anti-BCMA×CD3 scFv- Human IgG1 Fc Tag with a linear range of 0.08-3 ng/mL and cannot bind Human IgG1 Fc Protein, Tag Free (MALS verified) (Cat. No. FCC-H5214).
Human IgG1 Fc is used as an isotype control to verify the specific binding of anti-BCMA×CD3 scFv- Human IgG1 Fc Tag to Human BCMA protein.
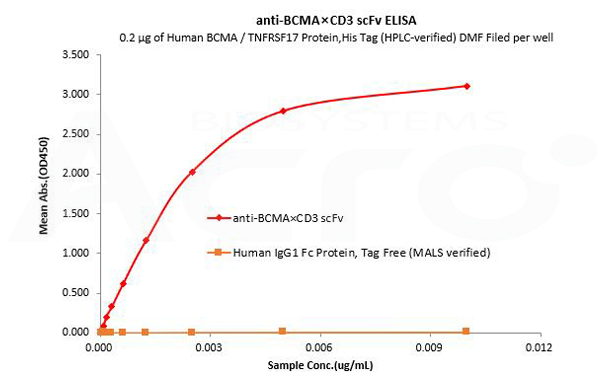
Human BCMA / TNFRSF17 Protein,His Tag (HPLC-verified) DMF Filed (Cat. No. BCA-H522y) can bind anti-BCMA×CD3 scFv- Human IgG1 Fc Tag with a linear range of 0.08-3 ng/mL and cannot bind Human IgG1 Fc Protein, Tag Free (MALS verified) (Cat. No. FCC-H5214).
>>Programmable half-life and anti-tumor effects of Nanobody
>>Increase or Decrease: The Two Therapeutic Strategies via FcRn-Mediated Mechanism
Authors:Hayes JM, Wormald MR , Rudd PM, Davey GP
Authors:Yu et al.
Authors:Ditza Levin et al.
This web search service is supported by Google Inc.
