Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Aneuro
As a leading manufacturer of recombinant proteins and other critical reagents for support in developing target therapeutics, vaccines, and diagnostics, ACROBiosystems employs an application-oriented development strategy, with a particular focus on product design, quality control, and solution-based support.
Aneuro is a new product line of ACRO that focuses and reflects dedicated efforts in neuroscience research. We aim to promote and facilitate neuroscience research by providing high-quality protein products and valuable new ideas.
Alzheimer's disease (AD) is a serious neurodegenerative disease. It was first proposed by the German doctor Alois Alzheimer in 1911 and was officially named by Kraeplin. The clinical symptoms of Alzheimer's disease are mainly manifested as memory impairment, mental decline, and impairment of motor balance. As an elderly-related disease, the increase in the elderly population also directly drives the market demand for AD treatment drugs. According to the World Alzheimer Report statistics, the number of AD patients worldwide will reach 131 million by 2050.
The pathological changes of AD are complex and diverse. Neuronal loss, synaptic disorders (e.g., synaptic loss and defects in protrusion plasticity), extracellular amyloid-beta (Aβ) deposition to form amyloid plaques, abnormally phosphorylated Tau protein to form intracellular neurofibrillary tangles are common in AD patients.
At present, the reasons for various pathological changes cannot be explained, and the pathogenesis is still unclear. There are three classic hypotheses on the pathogenesis of AD.
The Aβ cascade hypothesis is the dominant hypothesis in the pathogenesis of AD. The deposition of Aβ to form amyloid plaque is one of the main pathological features of AD. Aβ is generated from amyloid precursor protein (APP) by secretase degradation. There are two pathways for APP degradation, mainly involving three secretases, α-secretase, β-secretase (BACE), and γ-secretase. In the amyloid pathway, APP is cleaved by BCAE to generate sAPPβ protein, which is further cleaved by γ -secretase to generate Aβ polypeptides, including Aβ1-42, Aβ1-40, etc., and released into the extracellular domain, which eventually aggregates to form amyloid plaques, leading to the development of AD.
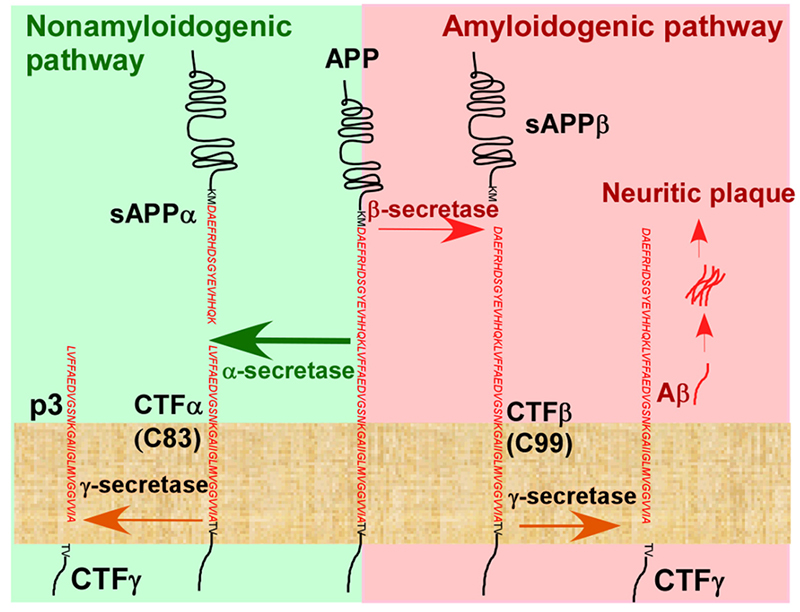
Hydrolysis pathway of APP in vivo
Binding to soluble, toxic aggregates of Aβ selectively to neutralize and eliminate it is thought to help alleviate neurodegenerative processes in AD. Based on this hypothesis, several drugs have been developed. Aducanumab is a representative drug targeting APP, a monoclonal antibody of Biogen, and was approved for marketing by the FDA on June 7, 2021.
Schematic diagram of monoclonal antibodies targeting APP
In addition, based on the same mechanism of action and target, Lilly's Donanemab and Roche's Gantenerumab are currently in clinical phase III, and Eisai and Biogen's Lecanemab are in the fast application stage of FDA. On February 7, 2022, Fierce Pharma ranked the most anticipated new drug launches for 2022, Donanemab and Ganteneruma ranked first and third.
Neurofibrillary tangles (NFTs) are another major pathological feature of AD patients. Under pathological conditions, excessive or abnormal phosphorylation of intracellular Tau protein makes it lose its biological activity of promoting microtubule assembly, causing microtubule depolymerization and disturbance of axonal operation, which leads to neuron degeneration and apoptosis of nerve cells, resulting in the occurrence of AD.
High expression of various phosphorylated kinases, including the glycogen synthase kinase 3β (GSK-3β), cyclin-dependent kinase 5 (CDK5), and tyrosine kinase, are the key factors of abnormal phosphorylation of Tau protein and are considered as potential drug targets for AD treatment.
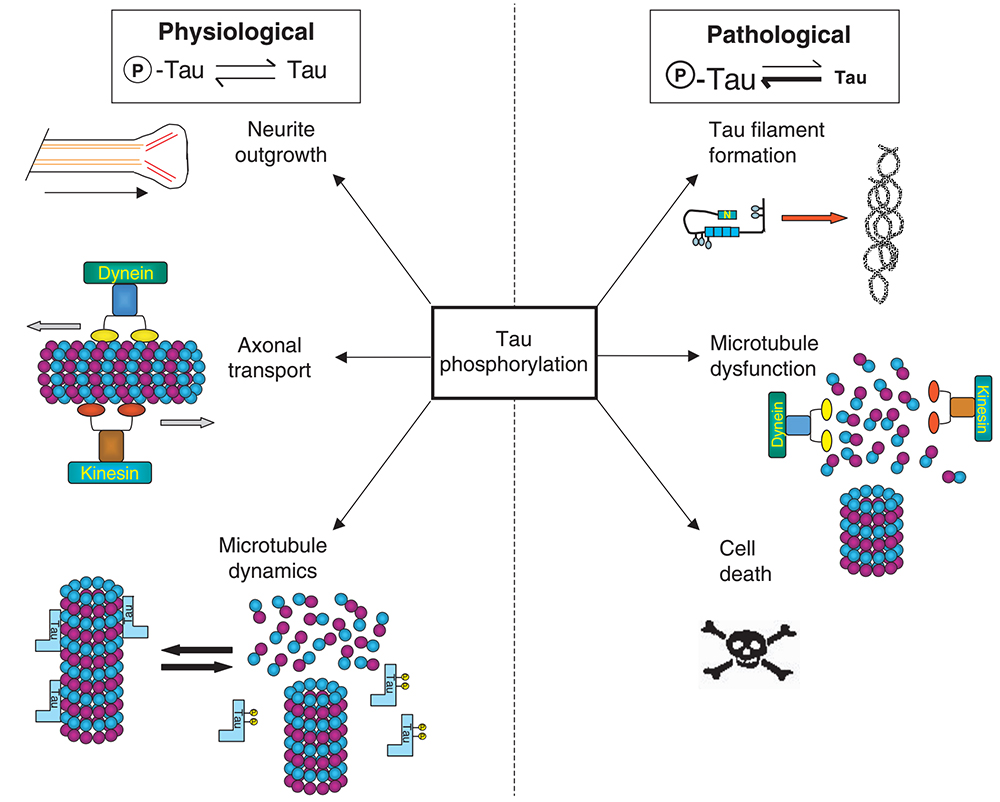
Abnormal phosphorylation of Tau protein
The cholinergic hypothesis was the first hypothesis to explain AD's pathogenesis.
Doucette et al. found severe loss of basal forebrain cholinergic neurons in AD patients, which led to decreased acetylcholine transferase (ChAT) activity for acetylcholine synthesis and severe depletion of presynaptic cholinergic transmitters, which led to cognitive function decline. The cholinergic hypothesis suggests that the decreased activity of Cholinesterase, including acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and ChAT, is the main cause of the decline in cholinergic levels.
Several Cholinesterase inhibitors for the treatment of AD have been approved by the FDA, such as donepezil hydrochloride and memantine hydrochloride/donepezil Hydrochloride. They are still available used as first-line clinical drugs for the treatment of mild to moderate AD. Even though these drugs play important roles in delaying the occurrence of AD, they cannot fundamentally treat AD due to their limited effects.
In addition to the three hypotheses of the Aβ cascade, abnormal phosphorylation of Tau protein and cholinergic, neuroinflammation, abnormal excitation of glutamate system, mitochondrial dysfunction, and other explanations for the pathogenesis of AD. The pathogenesis of AD is still under exploration in the scientific community.
In nearly 20 years, few new drugs have come to market except aducanumab. With the increasingly severe global aging situation, more drugs and therapies to improve the disease are urgently needed.
ACROBiosystems has developed Tau protein (TAU-441), amyloid precursor protein (APP), and β-secretase-1(BACE1) to support the development of AD therapeutic drugs.
| Molecule | Cat. No. | Species | Product Description |
|---|---|---|---|
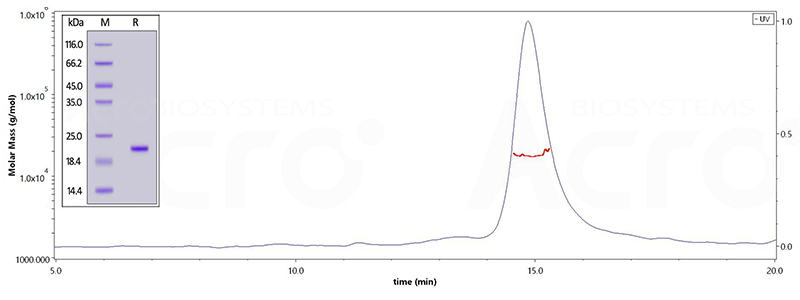
| Tau-441 | TAU-H51H3 | Human | Human Tau-441 / 2N4R Protein, His Tag |
| TAU-H51H5 | Human | Human Tau-441 / 2N4R (273-380) Protein, His Tag (MALS verified) | |
| TAU-H51H4 | Human | Human Tau-441 / 2N4R (241-380) Protein, His Tag (MALS verified) | |
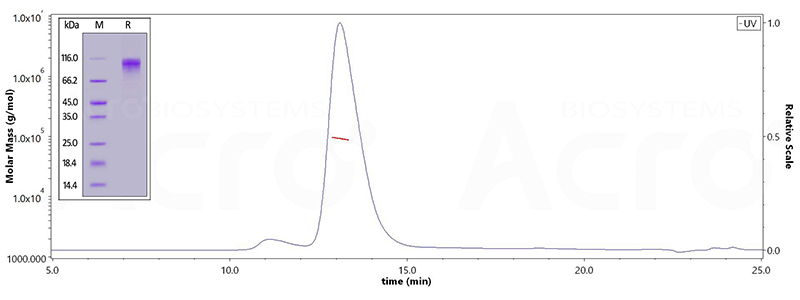
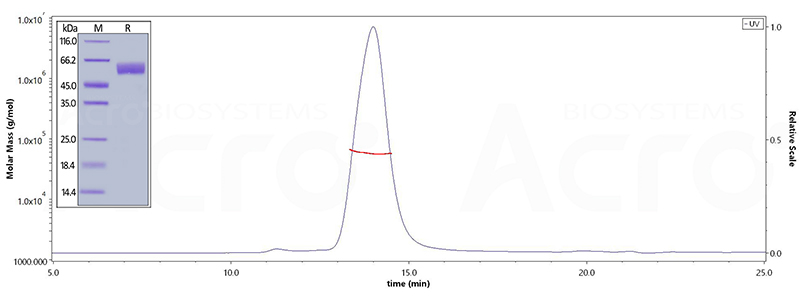
| BACE-1 | BA1-H5220 | Human | Human BACE-1 Protein, His Tag (active enzyme, MALS verified) |
| BA1-H5261 | Human | Human BACE-1 Protein, Fc Tag | |
| BA1-H5213 | Human | Human BACE-1 Protein, Tag Free (active enzyme, MALS verified) |
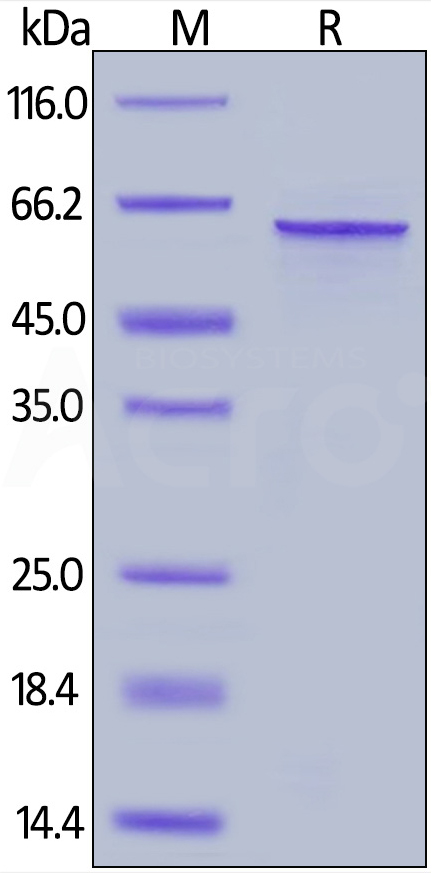
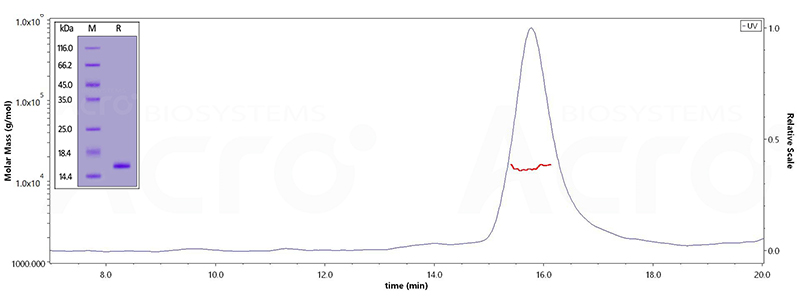
Human Tau-441 / 2N4R Protein, His Tag Human Tau-441, His Tag (Cat. No. TAU-H51H3) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 90%.
>>>Learn more about AneuRO: proteins for neuroscience
ACRO is developing more proteins for AD diagnosis and treatment. If you have any needs or more development suggestions, don't hesitate to get in touch with us.
1, Ju Y, Tam KY. Pathological mechanisms and therapeutic strategies for Alzheimer's disease. Neural Regen Res. 2022 Mar;17(3):543-549. doi: 10.4103/1673-5374.320970.
2, Zhang X, Song W. The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimers Res Ther. 2013 Oct 8;5(5):46. doi: 10.1186/alzrt211.
3, Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004 Nov 15;117(Pt 24):5721-9. doi: 10.1242/jcs.01558.
This web search service is supported by Google Inc.