Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Summary
Immune-Onc Therapeutics released their research of io-108, a novel clinical-stage myeloid checkpoint inhibitor targeting LILRB2 (ILT4), at the American Association for Cancer Research (AACR) 2022 Annual Meeting. IO-108 promotes innate and adaptive anti-cancer immunity in preclinical models, and it is well-tolerated in the ongoing Phase 1 study for the treatment of solid tumors.
The Leukocyte Immunoglobulin-like Receptor family (LILRs) contains members that can upregulate or downregulate immune cell activity and are critical to the regulation of immune response, such as cell migration, cell proliferation, phagocytosis, cytokine production, and secretion, chemical mediator production and secretion, and disease progression. LILRBs 1-5 are inhibitory LILRs, while LILRB1 (also known as LIR1, ILT2, and CD85J) and LILRB2 (also known as LIR2, ILT4, and CD85D) are the most extensively investigated.
LILRB1 is expressed in different types of leukemias and solid tumors. LILRB1 prevents primary cutaneous T cell lymphoma cell death and augments gastric tumor growth, while HLA-G/LILRB1 interaction inhibits neoplastic B cell proliferation.
LILRB2, also known as ILT4, is predominantly expressed on myeloid cells, including monocytes, dendritic cells, macrophages, and neutrophils. In solid tumors, LILRB2 can interact with various ligands such as HLA-G, Angiopoietin-like proteins (ANGPTLs), SEMA4A, and CD1d in a tumor microenvironment (TME) to promote myeloid tumor proliferation and tumor immune escape.
Human Leukocyte Antigen G (HLA-G) is a non-classical MHC class I molecule that plays an essential role in fetal-maternal tolerance, contributing to immune escape or anergy. It has been considered an immune checkpoint molecule recently.
There are currently 7 subtypes of the HLA-G protein, including four membrane-bound isoforms (HLA-G1, G2, G3, and G4) and three secreted soluble isoforms (HLA-G5, G6, and G7). Most research has focused on the full-length molecule HLA-G1 and its soluble isoform HLA-G4. HLA-G obstructs cytotoxic T cells, natural killer (NK) cells, and B cells, induces T cell anergy, regulates myeloid cells, and promotes T regulatory cells (Tregs). In addition, HLA-G is expressed on antigen-presenting cells (APCs).
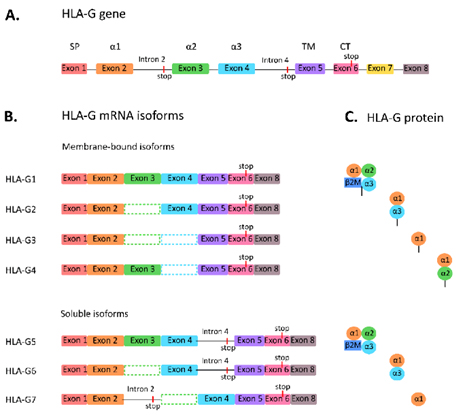
Figure 1. Subtypes of HLA-G
Studies have revealed that the HLA-G/LILRBs pathway has a broader impact than CTLA-4 and PD-1/PD-L1 regulated immune cells. The interaction of HLA-G with LILRB1 has been proven to inhibit the function of NK cells, dendritic cells, monocytes/macrophages, T cells, and B cells, therefore aiding tumor escape. Studies have also discovered that the interaction between LILRB2 expressed on solid tumor cells and HLA-G on adjacent tumor cells upregulates the expression of vascular endothelial growth factor C (VEGF-C) through the extracellular signal-regulated kinase (ERK) signaling pathway, causing metastasis, proliferation, and lymphangiogenesis. Furthermore, this interaction also upregulates B7-H3 expression via PI3K/AKT/mTOR signaling which encourages tumor escape. Interaction between LILRB1-expressing natural killer (NK) cells and HLA-G expressed on tumor cells and CD8+ T cells inhibit cytotoxicity, which promotes tumor escape. Expression of LILRB1 on naïve T cells also induces differentiation into Th2 cells and regulatory T cells (Tregs).
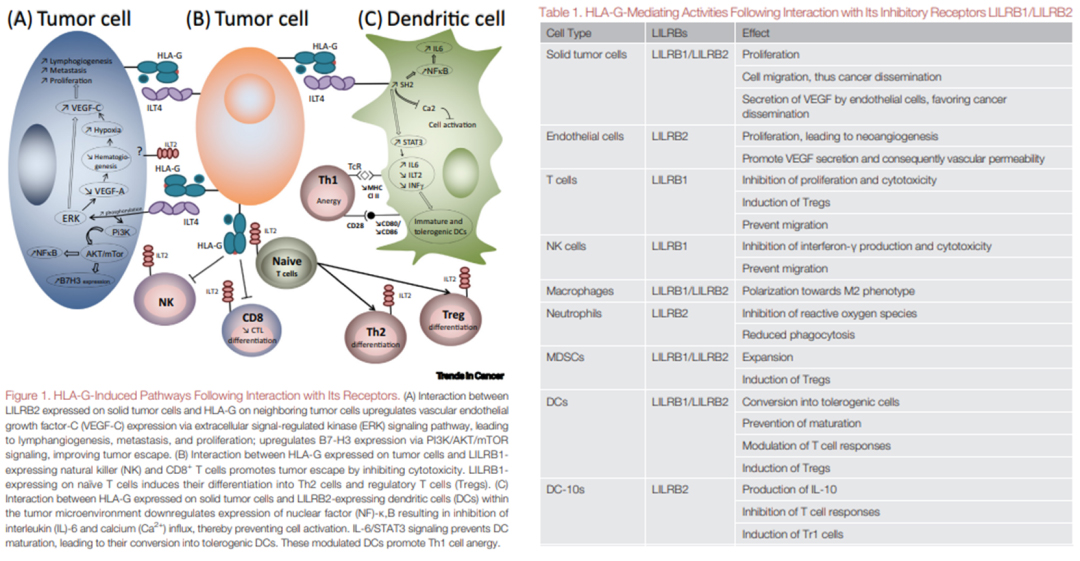
Figure 2. LILRBs interact with HLA-G to induce immunosuppression and promote tumor cell proliferation.
During tumor development, prolonged antigen stimulation causes antigen-specific T-cells to become dysfunctional, leading to the induction of depleted T cells. T cell exhaustion is characterized by poor effector function and expression of PD-1, TIM-3, LAG-3, CTLA-4, and TIGIT.
In certain tumor micro-environment, targeting CTLA-4 or PD-1 on T cells may be an effective option in certain cancer treatment. However, there is a risk of tumor-infiltrating T cells becoming refractory to checkpoint-blockade-mediated reactivation. Recently, a population of tumor-infiltrating CD8+ T cells expressing LILRB1, distinct from CD8+PD1+ T cells was identified in ccRCC patients. The CD8+LILRB1+-mediated cytotoxicity is prevented by HLA-G expression and neutralized by anti-HLA-G antibodies, validating the notion that targeting HLA-G can rejuvenate cancer-infiltrating exhausted T cells.
Studies have demonstrated that LILRB1/2 has more affinity to HLA-G1 dimer, results in increased inhibitory signals. The HLA-G1 monomer binds to a single LILRB1/2 molecule, whereas the HLA-G1 dimer can simultaneously interact with two LILRB1/2 molecules which amplify the LILRB-associated inhibition signals.
As the competition in cutting-edge drug research and development intensifies, selecting potential novel targets and forging a unique, visionary path can help innovators stand out. LILRB and HLA-G may become the next hot immunotherapy targets.
To facilitate related drug development, ACROBiosystems has released a high-quality HLA-G tetramer protein (Cat. No. HLG-H52E9) which can be used for immunosuppression research of LILRBs and HLA-G pathways.
![]() Based on ACROBiosystems’ unique biotin labeling technology, tetramer proteins have been developed. These tetramer proteins have been shown to amplify cell signals, stronger activities, and improved inhibitory effects on LILRB and HLA-G pathways.
Based on ACROBiosystems’ unique biotin labeling technology, tetramer proteins have been developed. These tetramer proteins have been shown to amplify cell signals, stronger activities, and improved inhibitory effects on LILRB and HLA-G pathways.
![]() The tetramer structure is verified by reducing and non-reducing electrophoresis and SEC-MALS. Purity is verified by SDS-PAGE (> 95%) and SEC-MALS (> 85%).
The tetramer structure is verified by reducing and non-reducing electrophoresis and SEC-MALS. Purity is verified by SDS-PAGE (> 95%) and SEC-MALS (> 85%).
![]() Neutralizing bioactivity is verified by ELISA assay. Furthermore, ACROBiosystems has also released a series of high-quality LILRBs proteins for immunization, antibody screening, species cross-verification, and other applications.
Neutralizing bioactivity is verified by ELISA assay. Furthermore, ACROBiosystems has also released a series of high-quality LILRBs proteins for immunization, antibody screening, species cross-verification, and other applications.
Furthermore, ACROBiosystems has also released a series of high-quality LILRBs proteins for immunization, antibody screening, species cross-verification, and other applications.
![]() Multiple species available: Human, Mouse, and Cynomolgus species are available to be used in species cross-verification.
Multiple species available: Human, Mouse, and Cynomolgus species are available to be used in species cross-verification.
![]() High-purity: purity is verified by SDS-PAGE (>95%) and MALS (>90%).
High-purity: purity is verified by SDS-PAGE (>95%) and MALS (>90%).
![]() Bioactivity is verified by ELISA and SPR assays; Affinity is verified by antibody and ligand binding assays.
Bioactivity is verified by ELISA and SPR assays; Affinity is verified by antibody and ligand binding assays.
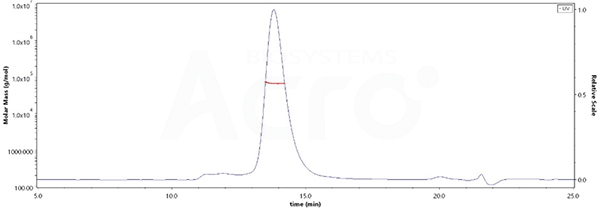
The purity of Human LILRB2, His Tag (Cat. No. LI2-H5220) is more than 90% and the molecular weight of this protein is around 58-78 kDa verified by SEC-MALS.
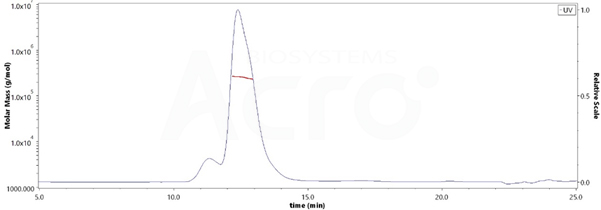
The purity of Human HLA-G RIIPRHLQL Tetramer Protein (Cat. No. HLG-H52E9) is more than 85% and the molecular weight of this protein is around 228-278 kDa verified by SEC-MALS.
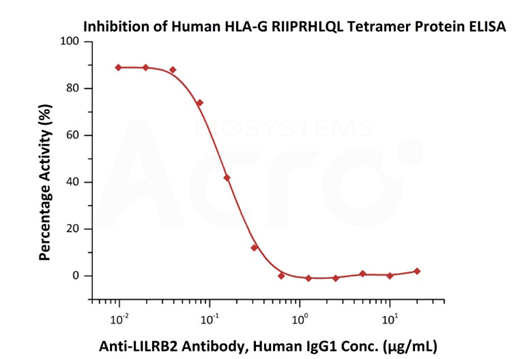
Serial dilutions of Anti-LILRB2 Antibody, Human IgG1 were added into Human LILRB2, Fc Tag (Cat. No. CDD-H5259) : Human HLA-G RIIPRHLQL Tetramer Protein (Cat. No. HLG-H52E9) binding reactions. The half maximal inhibitory concentration (IC50) is 0.1486 μg/mL (QC tested).
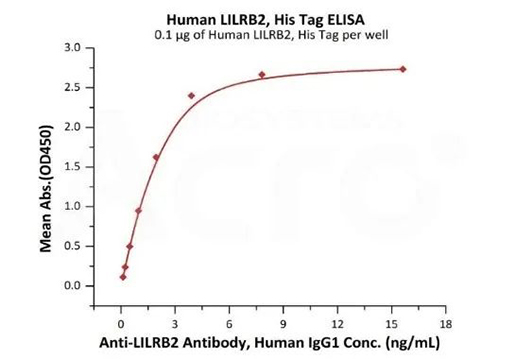
Immobilized Human LILRB2, His Tag (Cat. No. LI2-H5220) at 1 μg/mL (100 μL/well) can bind Anti-LILRB2 Antibody, Human IgG1 with a linear range of 0.5-4 ng/mL (QC tested).
This web search service is supported by Google Inc.