 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > [Inspiring Target] Therapeutic Potential of Complement in Inflammation, Cancer, and COVID-19 The complement system, also known as the complement cascade, is an essential part of the innate immune system and plays a crucial role in defense against microbial infection and clearance of immune complexes and injured cells. Under normal conditions, complement is tightly controlled by several humoral and cell surface proteins to avoid damage to autologous tissue. In subjects with autoimmune diseases or dysregulated regulatory proteins, severe inflammatory responses are triggered in many organs when complement is overactivated.
The intrinsic components of complement can be divided into four categories:
a. C1, C2, C4 in the classical activation pathway
b. Factor B, D, and P in the alternative activation pathway
c. MBL and serine protease in the mannan-binding lectin-activated pathway
d. C3, C5, C6, C7, C8, and C9 participating in the common terminal pathway
It is currently known that the complement system is composed of more than 30 soluble proteins, membrane-bound proteins, and complement receptors, which are widely distributed in circulation and tissues. They are synthesized and secreted by many cells under various stimuli, including cytokines and hormones.
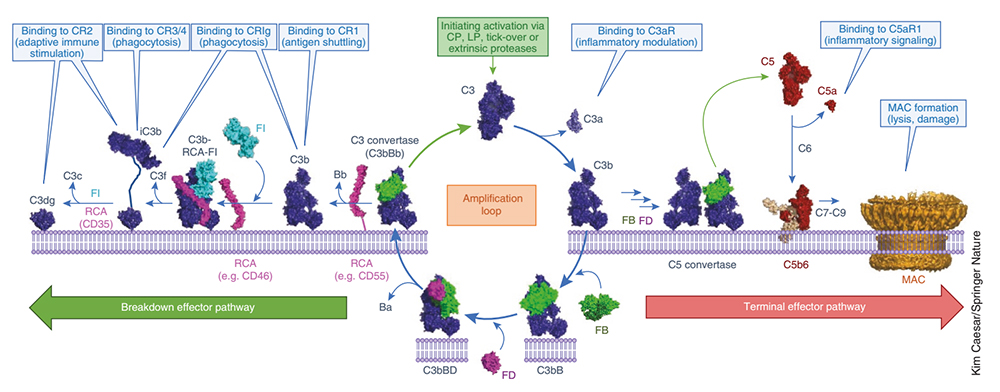
Molecular mechanism of C3-mediated complement activation, amplification, and effector molecule production
Despite the lack of specificity of the components of the acquired immune system, complement activates three pathways, the classical pathway, the lectin pathway, and the alternative activation pathway, enabling selective recognition of foreign pathogens and damaged endogenous cells.
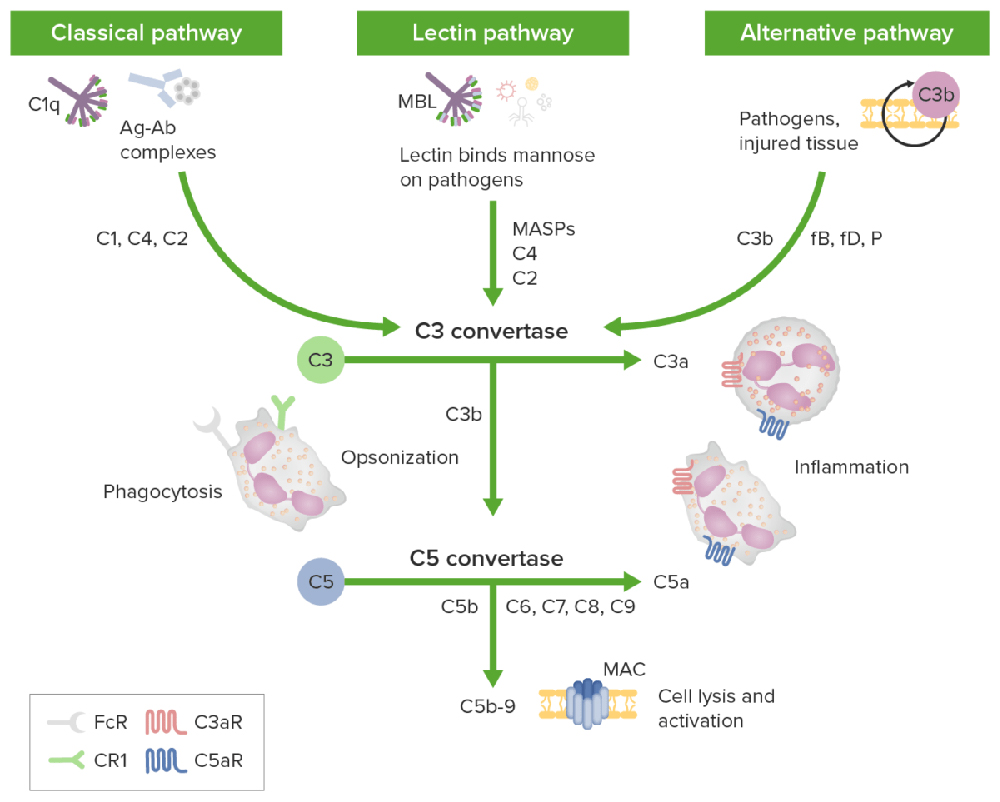
The three activation pathways of the complement cascade
· An antigen-antibody complex triggers the classic pathway, and the inactive C1 circulates as a complex of serum molecules, including Hexa-C1q molecules and two serine protease molecules, C1r and C1s. After binding to the antigen, the Fc fragment of an IgG or IgM antibody interacts with the collagen-like tail of C1q. This interaction leads to a conformational change and the sequential activation of C1r, C1s, C4, C2, and C3, forming a cascade enzymatic reaction process of C3 convertase (C4b2b) and C5 convertase (C4b2b3b).
· Mannan-binding lectin glycans recognize the lectin pathway, and the downstream effect is the same as the classical pathway.
· The alternative pathway is triggered by a mechanism independent of antibodies, involving factors B, D, and P, directly activating C3 by microorganisms or foreign substances, forming C3 and C5 convertases, and activating the activation of the complement cascade enzymatic reaction.
The above three complement pathways share a common terminal pathway: C5 can be cleaved into C5a and C5b under the action of C5 convertase. C5a is the strongest factor that appears in the early stage of acute infection and tissue damage inflammation and is recognized as a broad-spectrum inflammatory amplifier; C5b can form a membrane attack complex (MAC, C5b-9) with complement proteins C6, C7, C8, and C9, which directly lyses targeted pathogens or damaged autologous cells.
As a part of the innate immune system, the complement system is not adaptive throughout individual life, it has homeostatic importance in early development, but it is abnormally reactivated in an aging immune system. Therefore, complement is a key contributor to the pathological inflammatory process, including aging-related diseases.
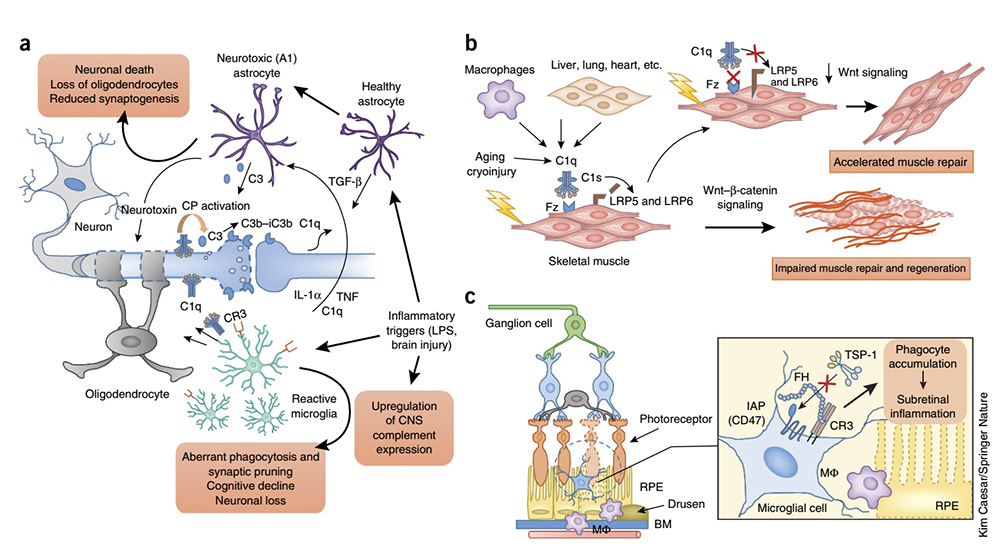
The role of complement in inflammation
Studies have revealed that complement drives and participates in chronic and aging-related inflammatory conditions, from the skin, kidney, and eye diseases to neuroinflammation and neurodegenerative diseases.
· The enrichment of C1q and C3 in the cortex of patients with refractory epilepsy correlates with abnormal microglial activity markers. The loss of inhibitory synapses may be responsible for the neuronal hyperexcitability observed in humans with epilepsy.
· Alzheimer's disease (AD) is the leading cause of age-related dementia worldwide and is marked by strong activation of astrocytes and microglia and deposition of complement on amyloid β plaques and tangles. Early complement components (C1q and C3) have been implicated in the phagocytic clearance of amyloid fibrils, while late complement effectors (C5a and MAC) have been implicated in inflammatory neuronal damage. Consistent with this, targeting C5aR1 improved pathological indicators in various transgenic AD models. Furthermore, C3 deficiency attenuated synaptic loss and cognitive decline in transgenic models of age-related AD pathology. (Click here to read Three hypotheses on the pathogenesis of Alzheimer's disease)
· Age-related macular degeneration (AMD) is a common ocular inflammatory disease and the leading cause of blindness in the elderly worldwide. Genome-wide association studies have identified FH polymorphisms as key susceptibility factors for AMD. The binding of FH to CR3 has been shown to inhibit the activation of the integrin-related receptor CD47 by thrombospondin-1, which is required for the homeostatic elimination of subretinal phagocytes. The AMD-associated FH variant H402Y enhanced this inhibitory effect, resulting in a pathological accumulation of subretinal phagocytes.
Complement plays a key role in determining tumor growth by regulating inflammation, T-cell immunity, vascularization, and tumor cell proliferation, migration, and invasive capabilities. Imbalanced complement activation in the tumor microenvironment triggers the release of pro-inflammatory cytokines from tumor cells and tumor-infiltrating immune cells, and local inflammation suppresses activation of effector T cells, creating an environment conducive to tumor growth. Complement activation products, especially C5a, promote angiogenesis, tumor cell migration, and invasion and metastasis of adjacent tissues.
Complement has been shown to regulate the differentiation and recruitment of myeloid-derived suppressor cells to tumor sites. Studies in animal models of melanoma, intestinal, cervical, and lung cancers demonstrate that C3aR and C5ar1 mediated pathways form pro-tumor via activation and polarization of innate immune cells, the release of pro-tumor factors, and inhibition of effector T cells environment.
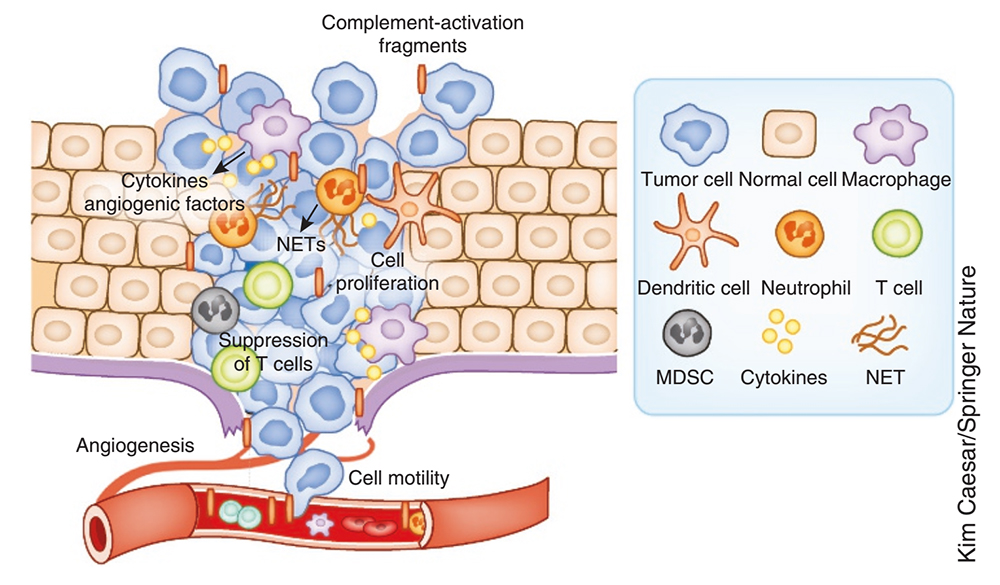
The role of complement in cancer
In melanoma and lung cancer models, combined blockade of complement and PD-L1 attenuated tumorigenesis more effectively than treatment alone, opening a new avenue for exploring complement inhibition combined with specific immunotherapy to activate patients' anticancer immunity. In addition, various monoclonal antibodies used clinically for cancer treatment have the function of activating the complement on the surface of tumor cells, thereby enhancing their killing and elimination effects.
Complement is a component of the innate immune response and pro-inflammatory response to viruses. Studies closely related to SARS-CoV-2 have found that activating complement component C3 can exacerbate acute respiratory distress syndrome (ARDS), and C3 inhibition may also alleviate inflammatory pulmonary complications of SARS-CoV-2 infection. C3 inhibition can simultaneously block C3a and C5a production, C3 activation, and IL-6 release in the lungs of alveolar macrophages or other cells expressing C3a receptors (C3aRs) and/or C5a receptors (C5aRs), thereby alleviating lung injury, it also suggested the possibility of combining C3 inhibitors with anti-IL-6 regimens. Lung biopsy samples from patients with severe COVID-19 showed extensive complement activation, characterized by C3a production and C3 fragmentation deposition, with markedly increased serum levels of C5a, and patients treated with anti-C5a antibodies experienced immediate clinical improvement. (Click here to read Therapeutic potential of IL-6 immunization in multiple fields continues to burst)
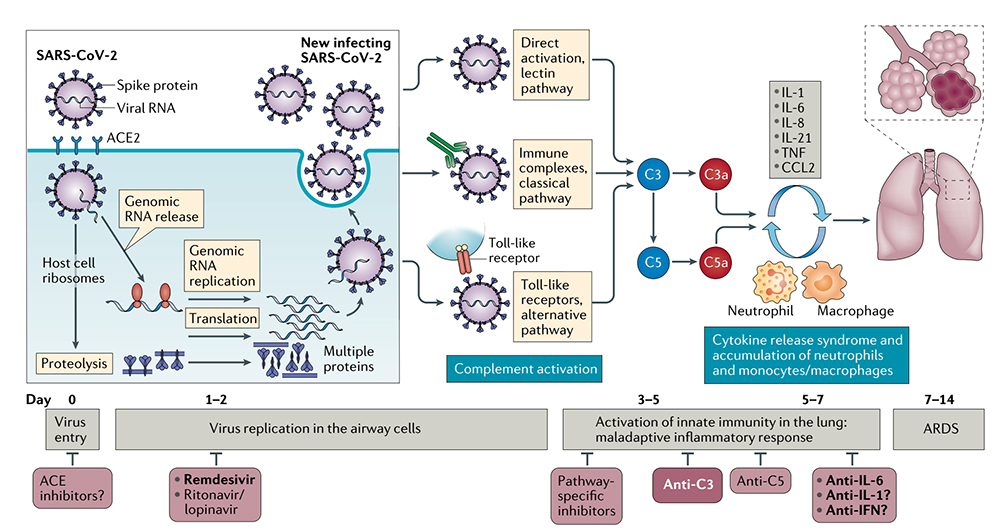
The role of targeted complement in SARS-CoV-2-associated lung injury
Complement activation may lead to maladaptive inflammatory responses in some severely ill COVID-19 patients. In these severe cases of COVID-19, C3 inhibition has the potential to be broadly applied to control ARDS and systemic inflammation in the kidney, brain, microvascular bed, and other vital organs. Therefore, inhibition of C3 or C5 may have therapeutic potential.
A better understanding of the complement's structure, function, and biological role will support our complement-based understanding to contribute to treating inflammation, neurodegenerative diseases, cancer, and COVID-19. ACROBiosystems has developed a series of recombinant complement proteins expressed by HEK293 Cells, including C2, C3, C5, C5a and complement factor D (CFD).
![]() Various species
Various species
![]() High purity verified by SDS-PAGE
High purity verified by SDS-PAGE
![]() Homogeneous structure verified by MALS
Homogeneous structure verified by MALS
![]() High biological activity verified by ELISA and cell-based assay
High biological activity verified by ELISA and cell-based assay
| Molecule | Cat. No. | Species | Product Description |
|---|---|---|---|
| Complement C2 | CO2-H52H7 | Human | Human Complement C2 Protein, His Tag (MALS verified) (active enzyme) |
| Complement C3 | CO3-H52H3 | Human | Human Complement C3 Protein, His Tag (MALS verified) |
| CO3-M52H4 | Mouse | Mouse Complement C3 Protein, His Tag (MALS verified) | |
| CO3-C52H5 | Cynomolgus | Cynomolgus Complement C3 Protein, His Tag (MALS verified) | |
| Complement C5 | CO5-H52Ha | Human | Human Complement C5 Protein, His Tag |
| CO5-H52Hx | Human | Human Complement C5 (R885H) Protein, His Tag | |
| CO5-H52H7 | Human | Human Complement C5 (w917s) Protein, His Tag | |
| CO5-M52H4 | Mouse | Mouse Complement C5 Protein, His Tag | |
| CO5-R52H5 | Rat | Rat Complement C5 Protein, His Tag | |
| CO5-C52Hx | Cynomolgus | Cynomolgus Complement C5 Protein, His Tag | |
| CO5-R52H4 | Rabbit | Rabbit Complement C5 Protein, His Tag | |
| Complement C5a | C5A-H5116 | Human | Human Complement C5a Protein, Tag Free |
| C5A-H525a | Human | Human Complement C5a Protein, Fc Tag (MALS verified) | |
| C5A-M51H4 | Mouse | Mouse Complement C5a Protein, His Tag | |
| C5A-C5118 | Cynomolgus | Cynomolgus Complement C5a Protein, Tag Free | |
| Complement Factor D | CFD-H52H8 | Human | Human Complement Factor D / CFD Protein, His Tag (active enzyme) |
| CFD-H5256 | Human | Human Complement Factor D / CFD Protein, Fc Tag | |
| CFD-R52H3 | Rhesus macaque / Cynomolgus | Rhesus macaque / Cynomolgus Complement Factor D / CFD Protein, His Tag (active enzyme) | |
| CFD-R5255 | Rhesus macaque | Rhesus macaque Complement Factor D / CFD Protein, Fc Tag |
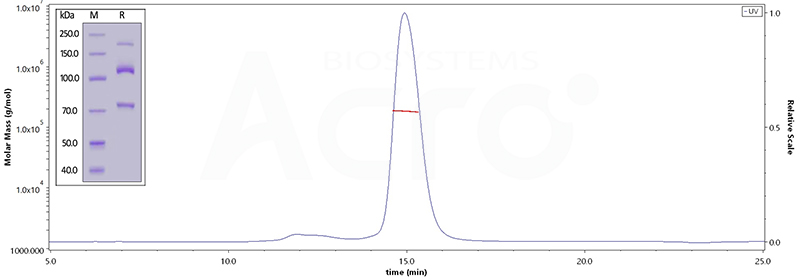
Human Complement C3, His Tag (Cat. No. CO3-H52H3) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%. The purity of Human Complement component 3, His Tag (Cat. No. CO3-H52H3), is more than 90%, and the molecular weight of this protein is around 168-204 kDa, verified by SEC-MALS.
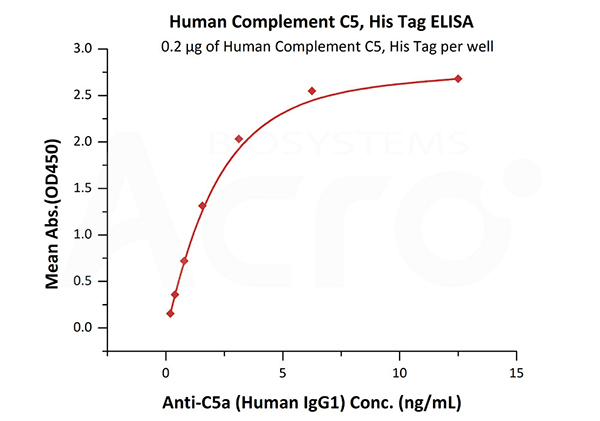
Immobilized Human Complement C5, His Tag (Cat. No. CO5-H52Ha) at 2 μg/mL (100 μL/well) can bind Anti-C5a (Human IgG1) with a linear range of 0.2-3 ng/mL (QC tested).
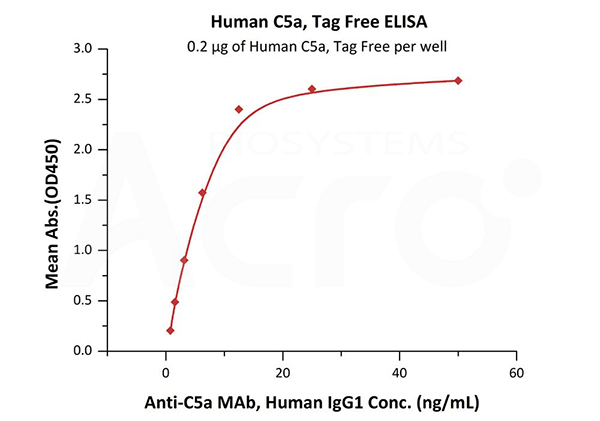
Immobilized Human Complement C5a, Tag Free (Cat. No. C5A-H5116) at 2 μg/mL (100 μL/well) can bind Anti-C5a MAb, Human IgG1 with a linear range of 0.8-13 ng/mL (QC tested).
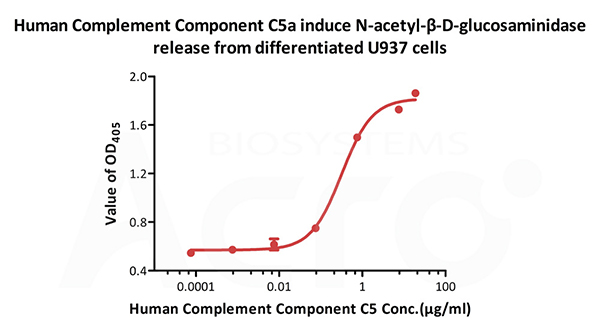
Human Complement C5a, Tag Free (Cat. No. C5A-H5116) induce N-acetyl-β-D-glucosaminidase release from differentiated U937 cells. The ED50 for this effect is 0.215-0.323 μg/mL.
ACROBiosystems provides a series of recombinant cytokines and their receptors, including interleukins, growth factors, tumor necrosis factors (TNFs), chemokines, and colony-stimulating factors (CSFs), interferons (IFNs), complement, etc. to accelerate your scientific research and drug development programs.
1. Hajishengallis, G., Reis, E., Mastellos, D. et al. Novel mechanisms and functions of complement. Nat Immunol 18, 1288–1298 (2017). https://doi.org/10.1038/ni.3858
2. https://www.lecturio.com/concepts/innate-immune-response/
3. Marina Noris, Giuseppe Remuzzi, Overview of Complement Activation and Regulation, Seminars in Nephrology(2013). https://doi.org/10.1016/j.semnephrol.2013.08.001.
4. Risitano, A.M., Mastellos, D.C., Huber-Lang, M. et al. Complement as a target in COVID-19?. Nat Rev Immunol 20, 343–344 (2020). https://doi.org/10.1038/s41577-020-0320-7
This web search service is supported by Google Inc.
