Background
With a portfolio of over 5,000 recombinant proteins and an industry-leading, scale-up ready protein development platform, ACROBiosystems has accumulated over 10 years of experience in developing recombinant proteins. Using this platform, our custom GMP-grade protein services are designed to ensure that our proteins are both structurally designed and validated for cellular therapies manufacturing. We take care to adhere strictly to the GMP guidelines with our comprehensive quality management system and quality controls, providing you with high-quality raw materials without disrupting your development process. Our custom GMP-grade protein service is a one-stop service based on your needs to maximize your therapy’s success. We offer two different developmental processes: converting our non-GMP protein products to GMP or developing a custom GMP-grade protein product from scratch.

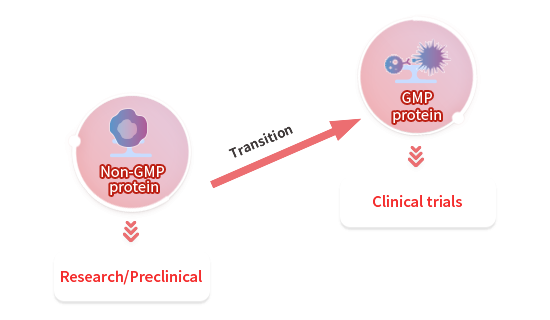
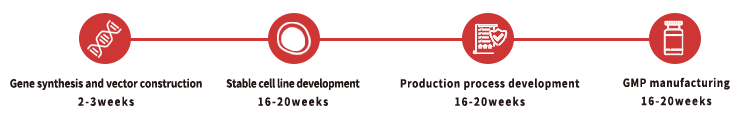
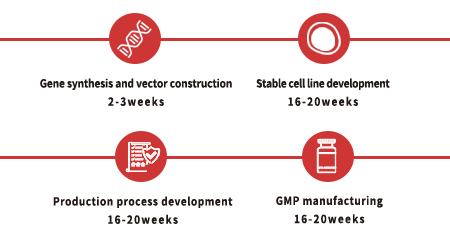
Custom GMP Protein Service Workflow
To facilitate a seamless transition from preclinical development to clinical phase, we recommend that you identify the appropriate key raw materials as early as possible during preclinical studies. For each of our custom projects, a dedicated project management team is provided to assist and support you throughout the entire process.
Immediate Development Needs: Use our non-GMP protein (Premium or RUO reagents) to save time, costs, and meet deadlines.
Medium-term Supply of Critical Reagents: Ensured large-scale production meets batch-to-batch consistency.
Long-term Product Development: Harness ACROBiosystems’ expertise across multiple platforms.
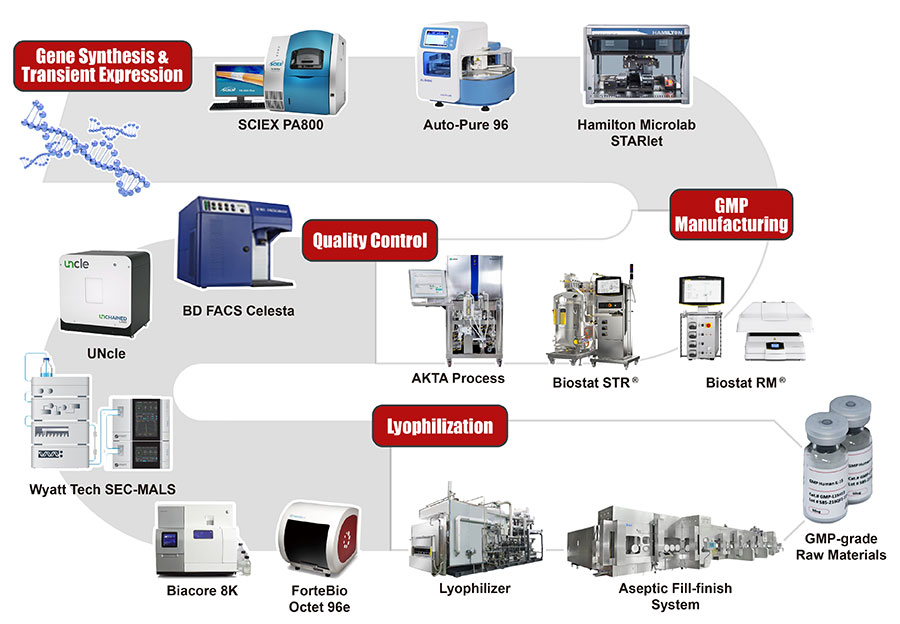
Industry-leading Custom GMP Service Development and Manufacturing Platform
Adapting our non-GMP proteins to GMP
GMP-grade protein products use the same clone, sequence, and expression system as our non-GMP grade proteins and have the same performance. As such, your raw materials can be easily transitioned to GMP-grade. From our extensive catalog, we can quickly convert our existing non-GMP grade raw materials to GMP-grade.
Starting from Scratch to GMP
We can also provide customized development services from scratch. After transitioning to GMP-grade, our products are ready to scale-up and match your demands through your clinical research and beyond.
SDS-PAGE, SEC-MALS, ELISA, SPR/BLI, FACS, Cell function, Sterility, Mycoplasma, Endotoxin
If you need a basic technical evaluation and quotation, please fill in our
GMP-grade custom protein service inquiry online and our technical staff will evaluate it accordingly. Evaluation results will be sent to the e-mail provided in the form. If GMP-grade is not needed but is planned in the future, please fill out our non-GMP grade form.
If you have any other questions or concerns, please feel free to send an e-mail directly to
custom@acrobiosystems.com or call our customer service number: +1 800-810-0816 for a consultation.

Platforms
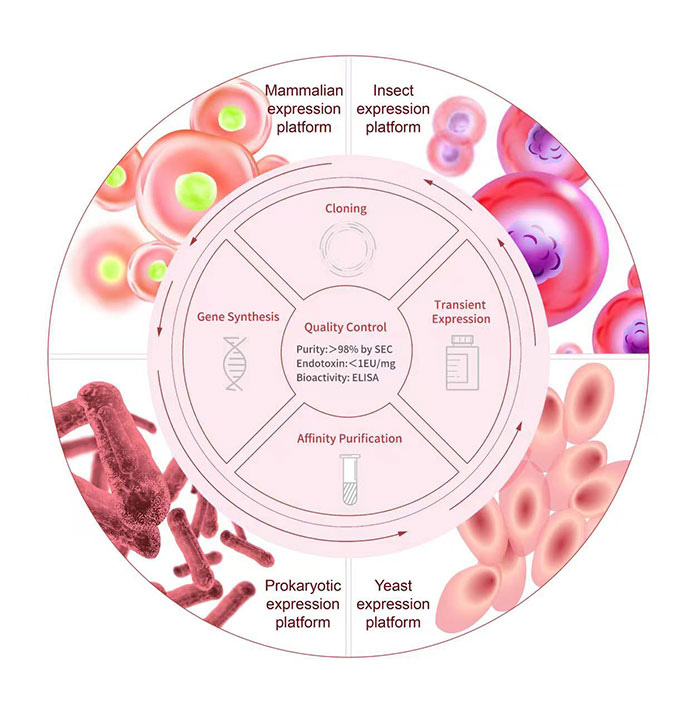
Industry-leading Recombinant Protein Development Platform
Here at ACROBiosystems, we have developed more than 5,000 recombinant protein products and accumulated more than 10 years of protein development and manufacturing experience. We provide a one-stop service starting from gene synthesis/vector construction to protein expression/purification. This includes all steps starting from protein design, codon optimization, gene synthesis, purification, to scale-up. Different expression systems, purification methods, and protein tags/labels can be selected based on your needs to maximize your therapy’s success.
Animal Component-free Processes (ACFP) under GMP-compliance
Our GMP facilities include ISO level 5 cleanrooms throughout the entire production process, they are available for online and on-site audits. Each step is automated or performed under sterile conditions to minimize external contamination risks. Production and purification procedures use equipment and media that are confirmed to be animal-free.
Comprehensive Quality Management Systems to Accelerate Clinical Processes
Since recombinant proteins are produced from biological systems, the manufacturing environment and processes makes it susceptible to batch-to-batch variability. The safety and effectiveness of key materials requires special attention in the manufacturing process and and quality control release. ACROBiosystems has several powerful protein analysis platforms that can perform comprehensive quality performance testings to ensure stable and reliable quality and strict control of exogenous contamination throughout the process to ensure safety (sterility, mycoplasma, exogenous viruses, endotoxins, etc.) in accordance with regulatory requirements.
GMP Grade Cytokines
ACROBiosystems is committed to the development of high-quality reagents that are used in the clinical stage of immune cell therapy drugs. Based on the GMP-grade quality management system platform, combined with the production specifications of cell therapy drugs, we have successfully developed a series of high-quality GMP-grade cytokines such as
IL-15,
IL-7,
IL-21. These products are produced with strict quality management and drug-level release testing standards. Our GMP-grade cytokines* can better assist the clinical research of immune cell therapy drugs and accelerate the global regulatory approval of biological products.
* ACROBiosystems GMP grade products are designed for research, manufacturing use, or ex vivo use. CAUTION: Not intended for human in vivo applications.


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now! Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions