Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Evaluating Inhaled Aerosol Vaccines: No-needle COVID-19 Protection? 
The COVID-19 epidemic has now entered its third year with its effects still being felt across the world. In many countries, there have been an alarming trend of waning vaccine efficacy paired with the emergence of variants with stronger immune escape and transmission ability. This consistent challenging of our pre-established immune barriers has driven the need for new, innovative vaccine modalities that overcome the hurdles of existing vaccination programs.
A recent breakthrough in COVID vaccine research is the regulatory approval of Covidencia™ Air from CanSinoBio, a first-in-class inhaled vaccine described to ‘effectively induce comprehensive immune protection in response to SARS-CoV-2 after just one breath’. This was swiftly followed by another inhaled aerosol vaccine by Bharat Biotech in India, indicated for ‘restricted use in emergency situations.’ With these new, innovative vaccine breakthroughs, what does this mean for the future of vaccine research?
The advent of this new administration modality directs our attention to the comparison between inhaled vaccines and conventional intramuscular vaccines. On one hand, intramuscular vaccines are designed to stimulate the systemic immune response, triggering the production of neutralizing antibodies such as IgGs. Contrary to this, aerosolized inhaled vaccines focus on simulating respiratory viral infection, leading to increased surface-level immune response and a quicker response to viral invaders.
It is important to note that aerosol-based vaccines have been introduced previously in the form of nasal vaccines. However, questions and concerns have been raised regarding its limited efficacy in initial studies. With these new, inhaled aerosol vaccines, these vaccines have been shown to have improved biodistribution deeper into the respiratory tract in comparison to nasal vaccines.
This increased biodistribution has shown to be induce a higher immune response and confer improved protective effects in preliminary studies.
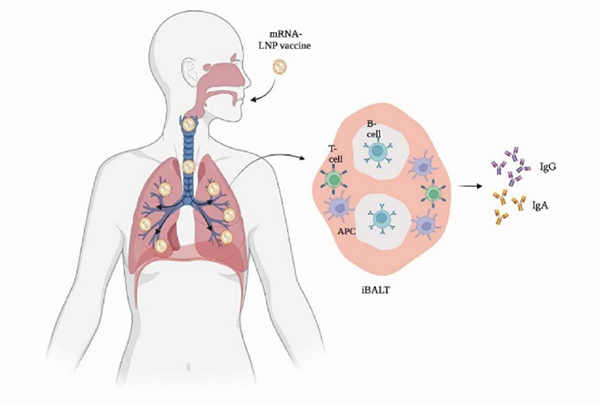
Figure 1. Mucosal immune response in the lung after vaccination through novel mRNA inhaled vaccine. 1
The advantages of an inhaled vaccines are undeniable, especially logistically, where these vaccines have lower dosages, conventional storage conditions, and have a wider distribution availability. This is heavily advantageous in resource-limited countries that may struggle with establishing and distributing newer, urgent vaccines. However, the evaluation of new vaccine modalities is incomplete, especially in regulatory settings. For intramuscular COVID-19 vaccines, the standard method in predicting vaccine efficacy is through the evaluation of neutralizing-antibody levels, while cellular immunity is evaluated by antigen-specific T cell response and cytokine secretion. Conversely, the proposed efficacy of inhaled vaccines stems from the stimulation of mucosal immune response alongside humoral and cellular responses. As such, the mucosal immune response has been highlighted as a critical immune response that could play a role in combating initial infections and should be evaluated. This is evidenced by the stimulation of the mucosal immune system, triggering the production of both IgGs and local IgAs. Produced by mucosal tissue, IgAs are responsible for pathogen neutralization and prevention of binding to mucosal tissue. Thus, when combating respiratory diseases such as COVID-19, IgA levels are increasingly of-interest when evaluating vaccine efficacy.
Overall, it might be easy to jump to the conclusion that inhaled vaccines will be the future route of administration for vaccines. Without the need for medical professionals, needles, and lower vaccine dosages, these inhaled and other aerosol-based vaccines are undoubtedly a significant breakthrough. According to a health-analytics company in London, there are currently around 100 aerosol-based COVID-19 vaccines are being developed in the world with around 20 of them reaching human clinical trials.2
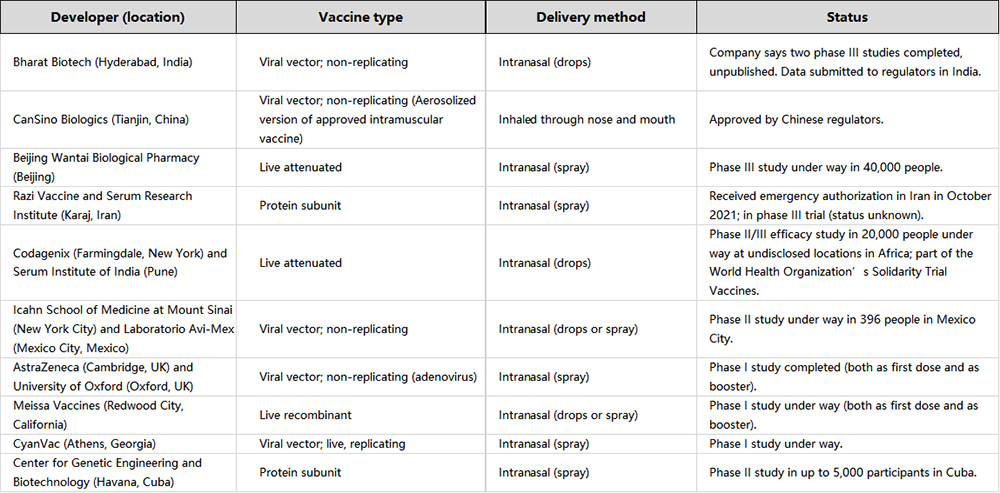
Despite the potential, conventional intramuscular vaccines are projected to retain its role as the front-line defense in vaccination. As the more mature method, intramuscular vaccines still provide strong immune responses to invading viruses. Most companies are developing inhaled vaccines as a complement or enhanced vaccine booster to improve the current state of immunity. This is supported by various studies that proposes a ‘prime-pull’ vaccination strategy which utilizes intramuscular vaccines to ‘prime’ the immune system with an inhaled booster to increase the immune defense.
Moreover, the effectiveness and potential of these inhaled vaccines needs to be further evaluated, especially as more inhaled vaccines obtain approval and widespread use. In theory, if these type of vaccines can help prevent infections and transmission, they could greatly impact the COVID-19 pandemic. Inhaled vaccines could boost a person’s “first line of defense” against the virus and have the potential to reduce onward transmission, Mike Ryan, the executive director of WHO Health Emergencies Programme, said at a press briefing. “But it remains to be seen.”
ACROBiosystems is still continuously monitoring the development of the COVID epidemic and vaccine research landscape.
To support supplemental and in-depth characterization of inhaled vaccines, a series of IgA antibody titer detection kits are offered across various variants and antibody isotype detection.
| Lineage | Cat. No. | Product Description |
|---|---|---|
| BA.2 | RAS-T104 | Anti-SARS-CoV-2 (BA.2) Antibody IgA Titer Serologic Assay Kit (Spike RBD) |
1. Jansen E., Frijlink H., Ruigrok M., et al. Are Inhaled mRNA vaccines safe and effective? A review of preclinical studies. Expert Opin Drug Deliv. 2022. 19(11):1471-1485.
2. COVID-19. https://www.airfinity.com/products/covid-19
This web search service is supported by Google Inc.