
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Chimeric antigen receptor T cell (CAR-T) therapies such as Kymriah and Yescarta, have been hailed as a significant breakthrough in treatment of hematological malignancies. However, the limitations of approved CAR-T therapies were evident when it came to treating solid tumors. Overcoming this hurdle has become one of the primary research goals for cell therapies, especially since solid tumors account for over 90% of all diagnosed cancers. Solid tumors, unlike its hematological counterpart, is comprised of a cellular milieu of various tumor, tissue and immune cells. This milieu, termed tumor microenvironment (TME), is both a physical and chemical barrier that prevents external CAR cells to penetrate and effect the tumor cells within. On one hand, the TME has a physical barrier with abnormal vascular structure and stromal composition. On the other, a variety of immunosuppressive cells are trapped within, secreting and maintaining a constant level of immune suppressive signals, making it difficult for cell therapies to infiltrate the tumor tissue. Furthermore, the tumor cell diversity within the TME makes it almost impossible for a tumor-specific antigen to be selected, manipulating the specificity of CAR-T cells against itself. In order to overcome these significant barriers, scientists have developed several strategies to overcome these obstacles to enable solid tumor targeting cell therapies.
Novel approaches to facilitate drug development include full-length transmembrane protein platforms, offering purified, high-quality TPs as off-the-shelf items for use in drug-target interaction analyses.
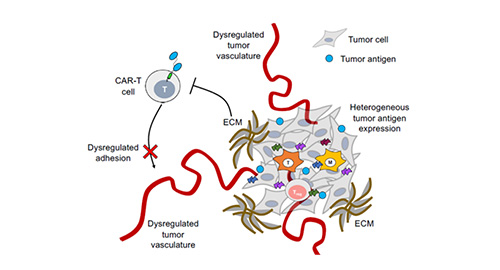
Figure 1. Poor penetration of CAR-T cells into the tumor microenvironment due to dysregulated tumor vasculature. 1
One of the key hurdles of current cell therapies is the decreased efficacy in infiltrating and targeting tumors within the TME. In order to improve infiltration of the tumor parenchyma, improved adhesion must first be achieved. A strategy targeting integrin αvβ3, a cellular receptor highly expressed on the surface of neovascular endothelial cells in solid tumors, was utilized to improve CAR T cell adhesion. This resulted in significantly improved elimination of αvβ3-positive tumor cells, inducing a complete elimination of melanoma lesions within a murine xenograft model. In other cases, chemotactic approaches were utilized, targeting chemokines secreted by tumor cells. The resulting chemokines bind to the chemokine receptors on the surface of modified T cells which promotes T cell infiltration into the TME.
The immunosuppressive environment fostered within the TME is a well-known and observed phenomena. This environment stems from the promotion of various immunosuppressive cytokines and cells while inhibiting the presence of immune activators. To combat this, several researchers have proposed the concept of over-expressing inflammatory cytokines such as interleukin-12,15, and 18. Referred to as ‘armored’ CAR-T cells, these cells are modified to include a self-expressing cytokine gene that helps modulate and offset the immunosuppressive environment within the TME.
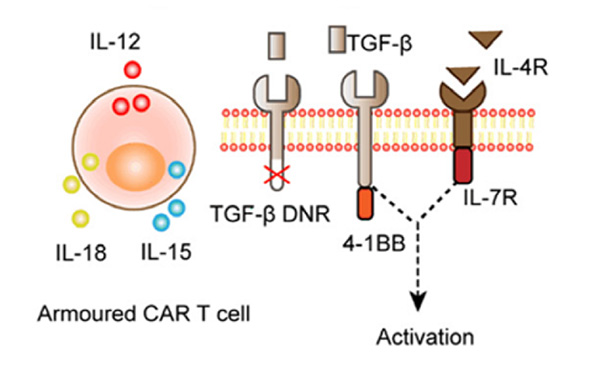
Figure2. Armored CAR-T cell that utilizes self-secretion of cytokines to promote immune activation within the TME. 2
Another immunosuppressive modality utilized by the TME is through the manipulation of immune checkpoints. These immunosuppressive receptors expressed on activated T cells have a high affinity to ligands expressed on the target cells, resulting in the promotion of immunosuppressive cellular signals, therefore adversely affecting T cell efficacy. To prevent this, gene editing technology has been used to reprogram T cells by ‘knocking out’ the immune checkpoint altogether. Electrotransfection encoding through sgRNA and Cas9 knockout of PD-1 on T cells resulted in a significantly enhanced cytotoxicity. A similar result occurred with the utilization of CRISPR/Cas9 to knockout CTLA-4 in cytotoxic T cells. This study reported tumor growth inhibition and subsequent tumor eradication through the elimination of immune-suppressive checkpoints.
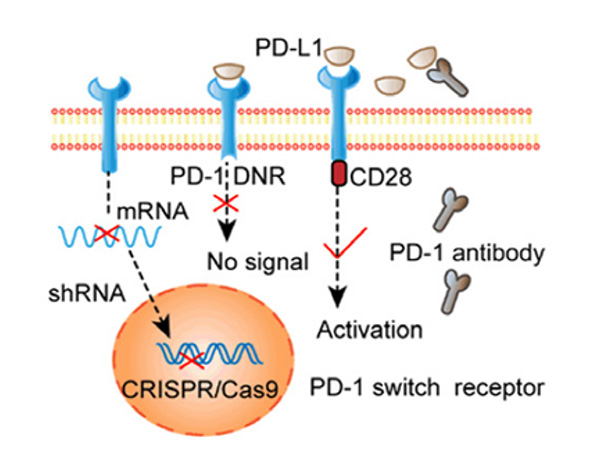
Figure 3. Selective activation of PD-1 signaling through electrotransfection encoded sgRNA and Cas9 knockout PD-1 T cells. 2
A common modality in combating solid tumors is through the specific targeting of antigens expressed on the tumor surface. However, for solid tumors, the heterogeneity of tumor cells leads to a diverse set of antigens, resulting in ineffective immune surveillance that promotes refractory and recurrent tumors. Currently, targets such as mesothelin (MSLN), GPC3, and HER2, are a commonality in tumor tissue reporting promising clinical results. In addition, tumor-associated fibroblasts (CAFs) have been also reported to promote angiogenesis and play a significant role in the pression and metatatsis of solid tumors.
The antigen specificity of CAR structure directly determines the precise activity and safety of modified T cells. However, for solid tumors, the heterogeneity of tumor antigens leads to ineffective immune surveillance, which leads to refractory and recurrent tumors. Currently, targets such as MSLN, GPC3 and HER2 have relatively high tumor tissue specificity in some solid tumors, and they have been used as CAR-T therapy targets, achieving good clinical results. In addition, tumor-associated fibroblasts (CAFs) have been reported to promote angiogenesis and play an important role in the progression and metastasis of solid tumors. FAP is a protein highly expressed in CAFs, representing a potential tumor growth and matrix reducing target for next generation CAR-T cells.
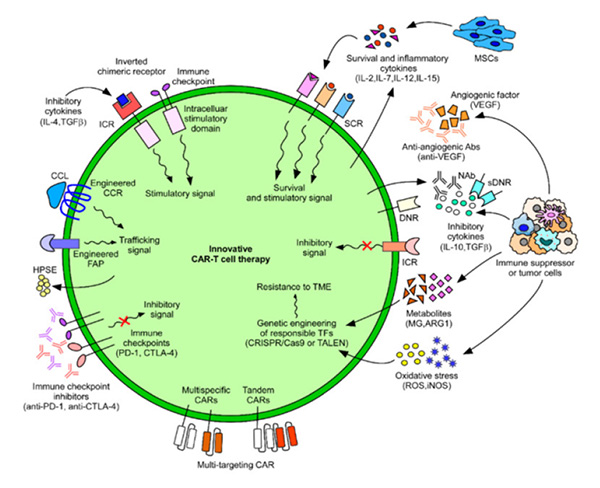
Figure 4. Innovative CAR-T cell therapies developed to combat cancers. 3
CAR-T cell therapy faces numerous difficulties in hurdling the challenge in combating solid tumors. However, several innovative strategies have been highlighted in recent years, reinvigorating the hopes for cell therapies and its subsequent potential. Through the optimization, modification, and research into the structure of CAR cells alongside adjuvant therapies, cell therapies are posed to have a breakthrough in the field of solid tumor therapies and to bring numerous benefits to clinical cancer patients.
To support the related research into CAR-T cell therapies, ACROBiosystems has developed a range of solid tumor-related target proteins, including MSLN, GPC3, FAP, HER2, EGFRvIII, covering different species and fluorescent types. These products can be used for immune screening, CAR expression, clinical PK and other studies to accelerate your cell therapy development.
References:
1. Hou A., Chen L., Chen Y. Navigating CAR-T cells through solid-tumor microenvironment. Nature Rev. Drug Discov. 2021. 20(7):531-550.
2. Huang M., Deng J., Gao L., Zhou J. Innovative strategies to advance CAR T cell therapies for solid tumors. Am J Cancer Res. 2020;10(7):1979-1992
3. Jo Y., Ali L.A., Hong C., et al. Innovative CAR-T Cell Therapy for Solid Tumor; Current Duel between Car-T Spear and Tumor Shield. Cancers (Basel). 2020;12(8):2087.
This web search service is supported by Google Inc.








