 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| GIP/GLP-1/FGF21 Fusion Protein treating NASH, T2DM, Hyperlipoidemia | NASH, T2DM, Hyperlipoidemia | Preclinical |
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| FG1-H82Q3 | Human | Biotinylated Human FGF-21 Protein, His,Avitag™ |  |
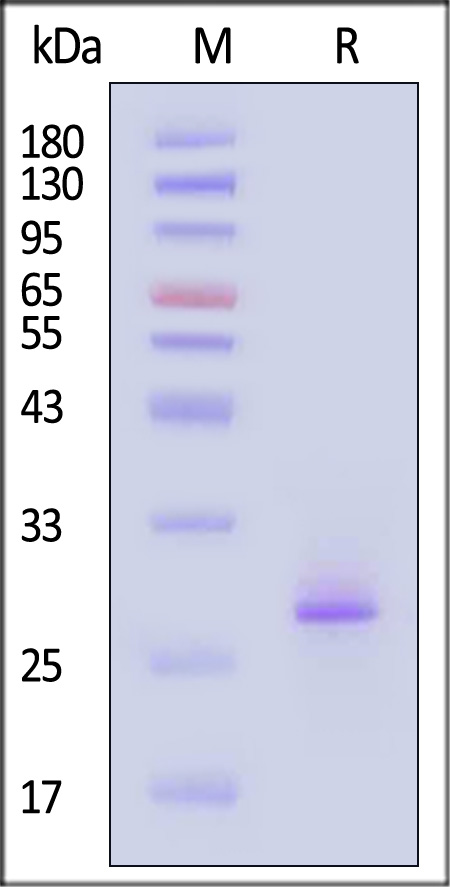
|
|
| FG1-H5243 | Human | Human FGF-21 Protein, His Tag |  |
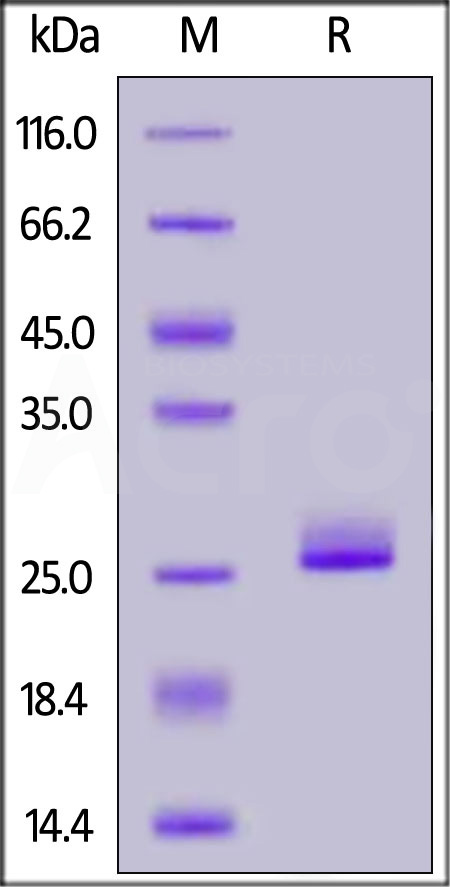
|
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Pegozafermin | BIO89-100; TEV-47948 | Phase 3 Clinical | Teva Pharmaceutical Industries Ltd | Hypertriglyceridemia; Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| Efruxifermin | AKR-001; AMG-876; EFX; Fc-FGF21(RGE) | Phase 3 Clinical | Amgen Inc | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus | Details |
| Recombinant human FGF21 Fc fusion protein(Anyuan Pharmaceutical) | AP-025 | Phase 2 Clinical | Anyuan Pharmaceutical Technology (Shanghai) Co Ltd | Hypertriglyceridemia; Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| HEC-88473 | HEC-88473; HEC88473 | Phase 2 Clinical | Dongguan Hec Taigen Biopharmaceuticals Co Ltd | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Hepatitis; Obesity | Details |
| YH-25724 | YH-25724; YH25724 | Phase 1 Clinical | Yuhan Corp | Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| AP-026 | AP-026; TQA-2226; TQA2226; AP026 | Phase 1 Clinical | Anyuan Pharmaceutical Technology (Shanghai) Co Ltd | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Obesity; Hyperlipidemias; Overweight | Details |
| Pegozafermin | BIO89-100; TEV-47948 | Phase 3 Clinical | Teva Pharmaceutical Industries Ltd | Hypertriglyceridemia; Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| Efruxifermin | AKR-001; AMG-876; EFX; Fc-FGF21(RGE) | Phase 3 Clinical | Amgen Inc | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus | Details |
| Recombinant human FGF21 Fc fusion protein(Anyuan Pharmaceutical) | AP-025 | Phase 2 Clinical | Anyuan Pharmaceutical Technology (Shanghai) Co Ltd | Hypertriglyceridemia; Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| HEC-88473 | HEC-88473; HEC88473 | Phase 2 Clinical | Dongguan Hec Taigen Biopharmaceuticals Co Ltd | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Hepatitis; Obesity | Details |
| YH-25724 | YH-25724; YH25724 | Phase 1 Clinical | Yuhan Corp | Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| AP-026 | AP-026; TQA-2226; TQA2226; AP026 | Phase 1 Clinical | Anyuan Pharmaceutical Technology (Shanghai) Co Ltd | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Obesity; Hyperlipidemias; Overweight | Details |
This web search service is supported by Google Inc.
