 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CD6-R52H9 | Rat | Rat B7-2 / CD86 Protein, His Tag |  |
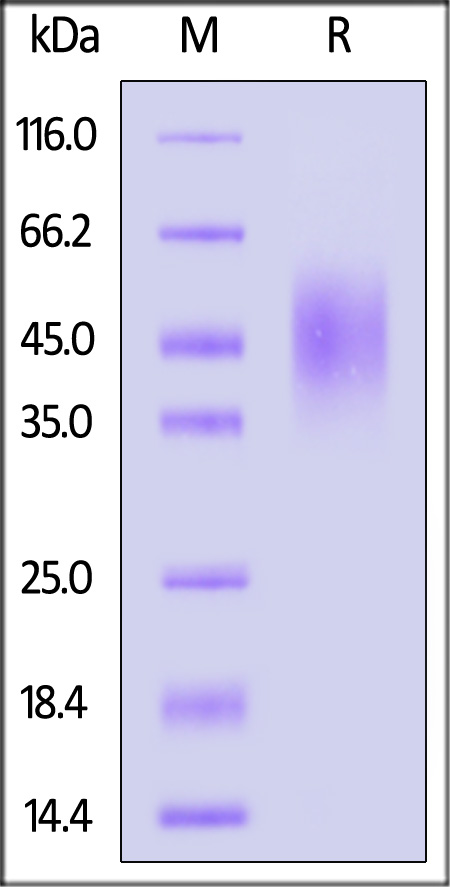
|
|
| CD6-H82F5 | Human | Biotinylated Human B7-2 / CD86 Protein, Fc,Avitag™, premium grade |  |
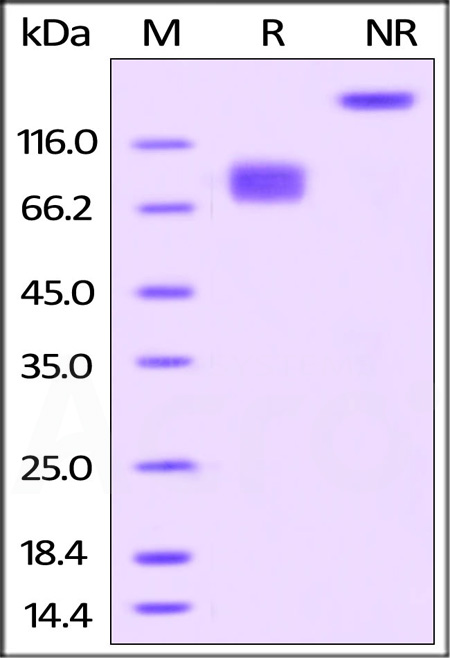
|
|
| CD6-M52H0 | Mouse | Mouse B7-2 / CD86 Protein, His Tag (MALS verified) |  |
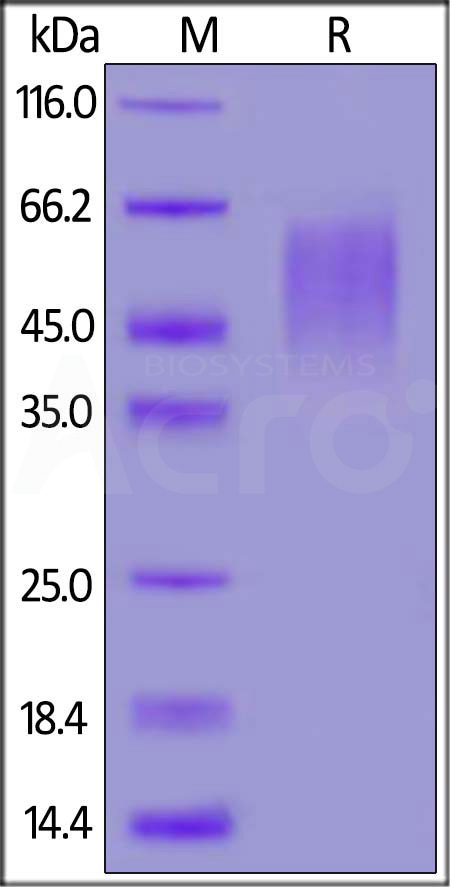
|
|
| CD6-M5251 | Mouse | Mouse B7-2 / CD86 Protein, Fc Tag (MALS verified) |  |
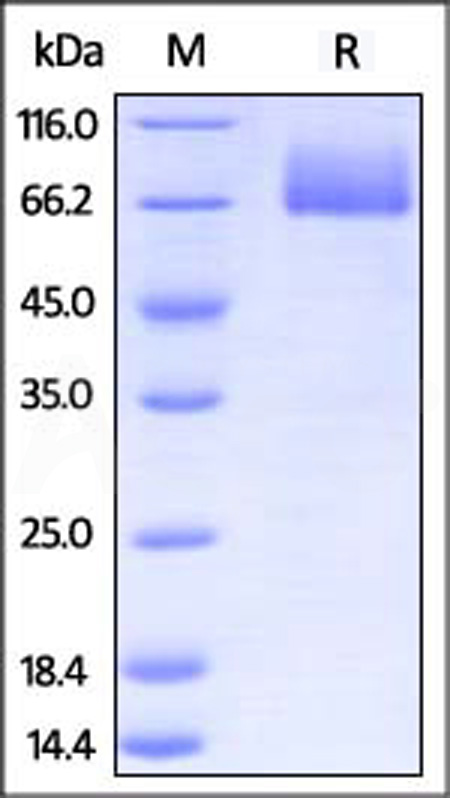
|
|
| CD6-C5254 | Cynomolgus / Rhesus macaque | Cynomolgus / Rhesus macaque B7-2 / CD86 Protein, Fc Tag |  |
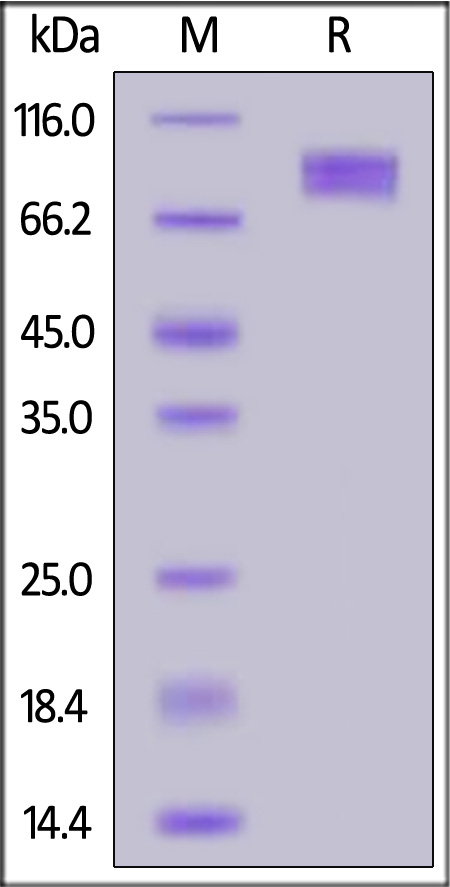
|
|
| CD6-C52H5 | Cynomolgus / Rhesus macaque | Cynomolgus / Rhesus macaque B7-2 / CD86 Protein, His Tag (MALS verified) |  |

|
|
| CD6-H5223 | Human | Human B7-2 / CD86 Protein, His Tag (MALS verified) |  |
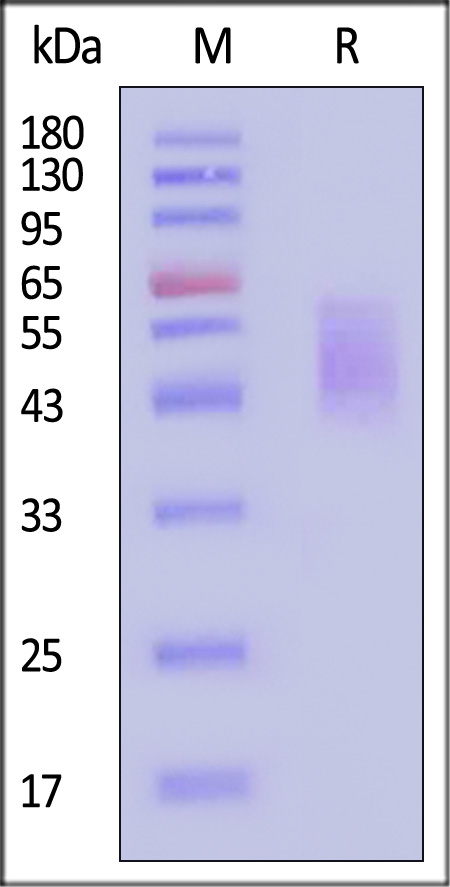
|
|
| CD6-H5257 | Human | Human B7-2 / CD86 Protein, Fc Tag, premium grade |  |
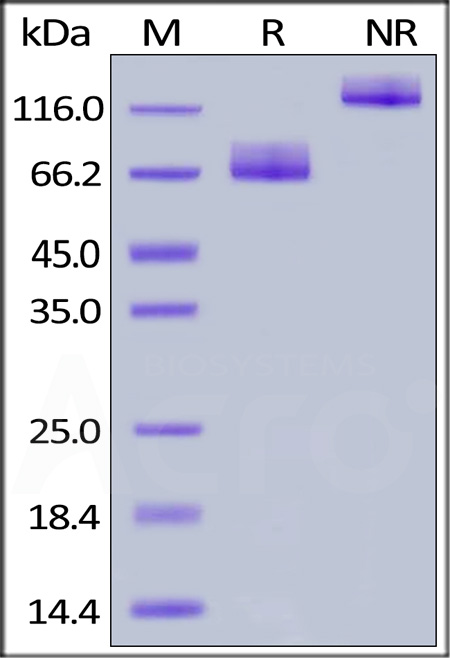
|
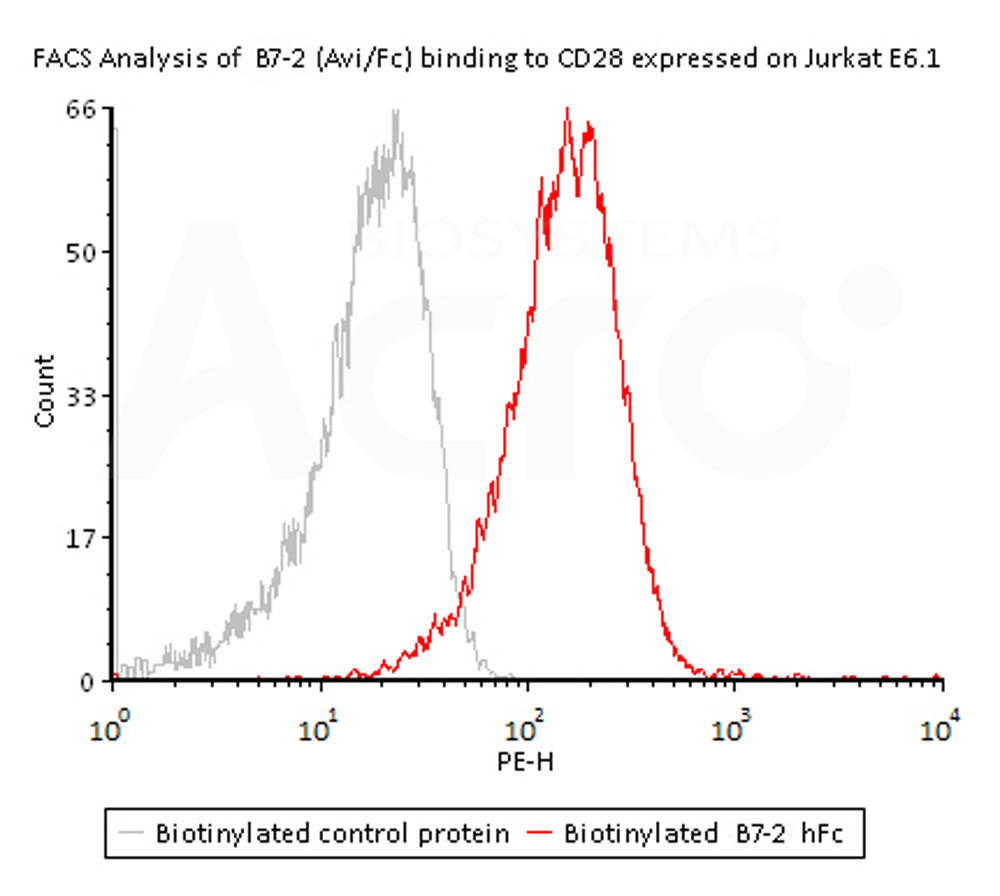
Flow Cytometry assay shows that Biotinylated Human B7-2, Fc,Avitag, premium grade (Cat. No. CD6-H82F5) can bind to CD28 expressed on Jurkat E6.1 cells. The concentration of B7-2 used is 1.5 μg/mL (Routinely tested).
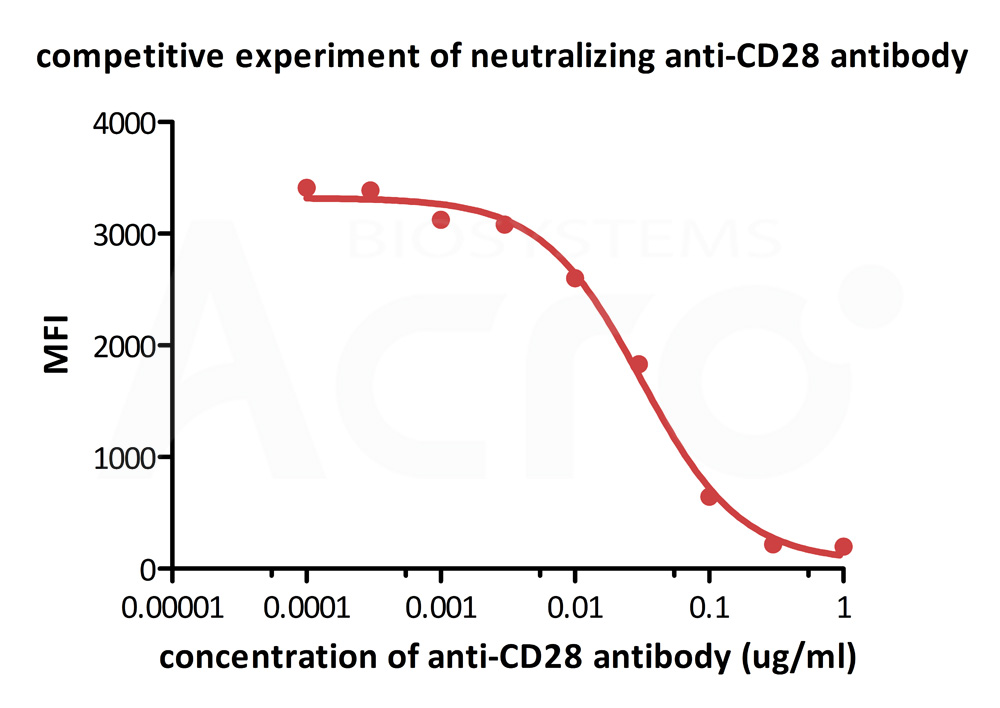
FACS analysis shows that the binding of Biotinylated Human B7-2, Fc,Avitag, premium grade (Cat. No. CD6-H82F5) to CD28 expressed on Jurkat E6.1 was inhibited by increasing concentration of neutralizing Anti-CD28 antibody. The concentration of B7-2 used is 1.5 μg/mL. The IC50 is 0.031 μg/mL (Routinely tested).
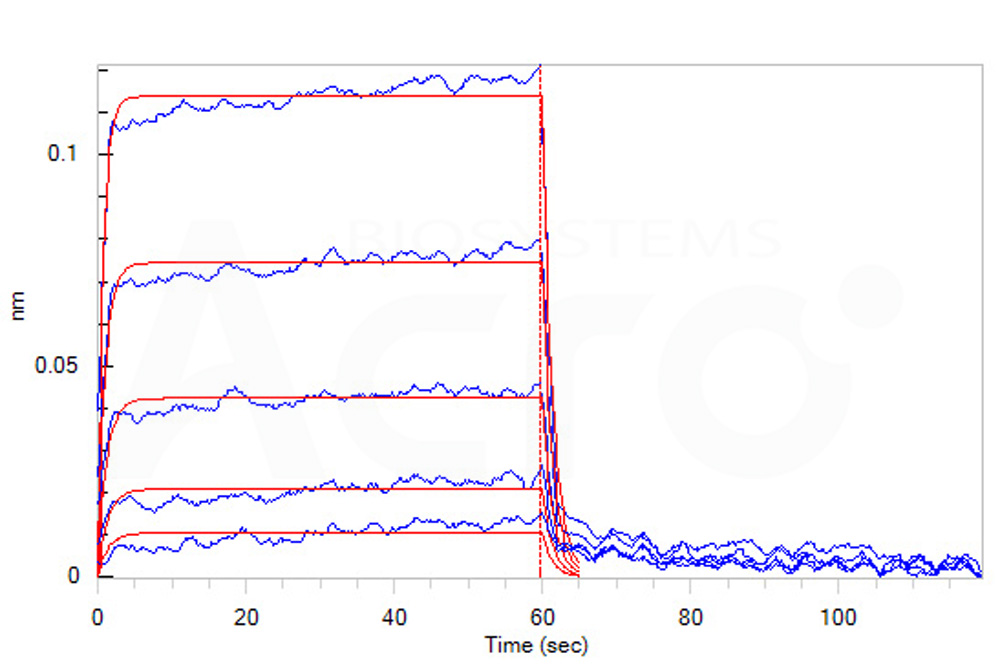
Loaded Cynomolgus / Rhesus macaque B7-2, Fc Tag (Cat. No. CD6-C5254) on Protein A Biosensor, can bind Human / Cynomolgus / Rhesus macaque CD28, His Tag with an affinity constant of 11 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Belatacept | LEA29Y; LEA-029; BMS-224818; L104EA29YIg | Approved | Bristol-Myers Squibb Company | Nulojix | United States | Rejection of renal transplantation | Bristol-Myers Squibb Company | 2011-06-15 | Rejection of renal transplantation; Diabetes Mellitus, Type 1; Proteinuria; Pancreatic neuroendocrine tumors (pNET); Arthritis, Rheumatoid; Delayed Graft Function; Rejection of organ transplantation; Rejection in heart transplantation; Kidney Failure, Chronic | Details |
| Abatacept | ONO-4164SC; BMS-188667SC; ONO-4164; BMS-188667 | Approved | Bristol-Myers Squibb Company | Orencia, 恩瑞舒 | United States | Arthritis, Rheumatoid | Bristol-Myers Squibb Company | 2005-12-23 | Arthritis, Psoriatic; Uveitis; Inflammation; Granulomatous Disease, Chronic; Urticaria; Asthma; Sjogren-Larsson Syndrome; Cardiovascular Diseases; Colitis, Ulcerative; Muscular Diseases; Glomerulosclerosis, Focal Segmental; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Anemia, Aplastic; Nephrosis, Lipoid; Mucopolysaccharidosis I; Shwachman-Diamond Syndrome; Scleroderma, Diffuse; Common Variable Immunodeficiency; Arthralgia; Arthritis; Myocarditis; Eye Diseases; Lymphohistiocytosis, Hemophagocytic; Leukocyte-Adhesion Deficiency Syndrome; Nephrotic Syndrome; Crohn Disease; Anemia, Sickle Cell; Thrombasthenia; Behcet Syndrome; Vitiligo; Kostmann Syndrome; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Arthritis, Rheumatoid; Giant Cell Arteritis; Immunoglobulin G4-Related Disease; Alopecia Areata; Dermatomyositis; Myositis; Multiple Sclerosis, Relapsing-Remitting; Myasthenia Gravis; Anemia, Diamond-Blackfan; Granulomatosis with Polyangiitis; Hematologic Diseases; Polychondritis, Relap | Details |
| Belatacept | LEA29Y; LEA-029; BMS-224818; L104EA29YIg | Approved | Bristol-Myers Squibb Company | Nulojix | United States | Rejection of renal transplantation | Bristol-Myers Squibb Company | 2011-06-15 | Rejection of renal transplantation; Diabetes Mellitus, Type 1; Proteinuria; Pancreatic neuroendocrine tumors (pNET); Arthritis, Rheumatoid; Delayed Graft Function; Rejection of organ transplantation; Rejection in heart transplantation; Kidney Failure, Chronic | Details |
| Abatacept | ONO-4164SC; BMS-188667SC; ONO-4164; BMS-188667 | Approved | Bristol-Myers Squibb Company | Orencia, 恩瑞舒 | United States | Arthritis, Rheumatoid | Bristol-Myers Squibb Company | 2005-12-23 | Arthritis, Psoriatic; Uveitis; Inflammation; Granulomatous Disease, Chronic; Urticaria; Asthma; Sjogren-Larsson Syndrome; Cardiovascular Diseases; Colitis, Ulcerative; Muscular Diseases; Glomerulosclerosis, Focal Segmental; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Anemia, Aplastic; Nephrosis, Lipoid; Mucopolysaccharidosis I; Shwachman-Diamond Syndrome; Scleroderma, Diffuse; Common Variable Immunodeficiency; Arthralgia; Arthritis; Myocarditis; Eye Diseases; Lymphohistiocytosis, Hemophagocytic; Leukocyte-Adhesion Deficiency Syndrome; Nephrotic Syndrome; Crohn Disease; Anemia, Sickle Cell; Thrombasthenia; Behcet Syndrome; Vitiligo; Kostmann Syndrome; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Arthritis, Rheumatoid; Giant Cell Arteritis; Immunoglobulin G4-Related Disease; Alopecia Areata; Dermatomyositis; Myositis; Multiple Sclerosis, Relapsing-Remitting; Myasthenia Gravis; Anemia, Diamond-Blackfan; Granulomatosis with Polyangiitis; Hematologic Diseases; Polychondritis, Relap | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ORCA-010 | ORCA-010 | Phase 2 Clinical | Orca Therapeutics Bv | Prostatic Neoplasms | Details |
| Chimeric antigen receptor T-cell therapy (Hunan Zhaotai Yongren Biotech) | Z-CTLs | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Abatacept biosimilar (Dr. Reddy's Laboratories) | DRL-AB | Phase 1 Clinical | Dr.Reddy's Laboratories Ltd | Arthritis, Rheumatoid | Details |
| Recombinant human CTLA-4-FC fusion protein(Beijing Vdjbio) | Phase 1 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid | Details | |
| ORCA-010 | ORCA-010 | Phase 2 Clinical | Orca Therapeutics Bv | Prostatic Neoplasms | Details |
| Chimeric antigen receptor T-cell therapy (Hunan Zhaotai Yongren Biotech) | Z-CTLs | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Abatacept biosimilar (Dr. Reddy's Laboratories) | DRL-AB | Phase 1 Clinical | Dr.Reddy's Laboratories Ltd | Arthritis, Rheumatoid | Details |
| Recombinant human CTLA-4-FC fusion protein(Beijing Vdjbio) | Phase 1 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid | Details |
This web search service is supported by Google Inc.
