 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| ILR-M5254 | Mouse | Mouse IL-10 R alpha / CD210 Protein, Fc Tag |  |
|
|
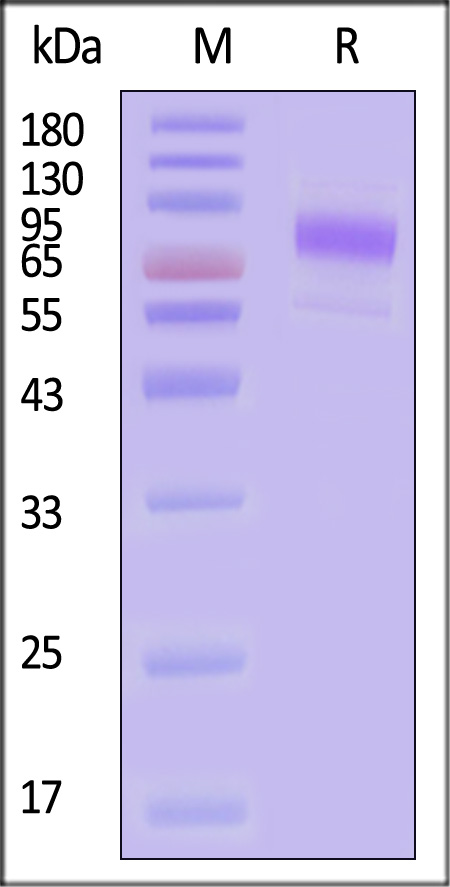
| ILR-H525a | Human | Human IL-10 R alpha / CD210 Protein, Fc Tag (MALS verified) |  |
|
|
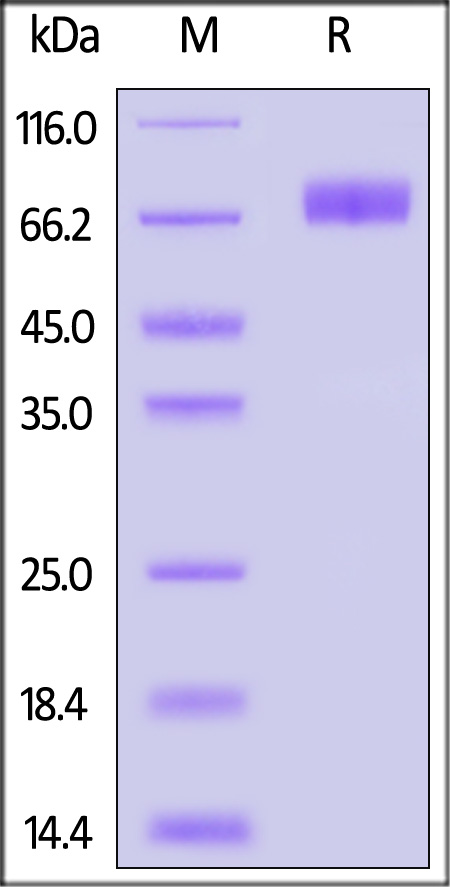
| ILR-C5256 | Cynomolgus | Cynomolgus IL-10 R alpha / CD210 Protein, Fc Tag |  |
|
|
| ILR-H82F6 | Human | Biotinylated Human IL-10 R alpha / CD210 Protein, Fc,Avitag™ (MALS verified) |  |
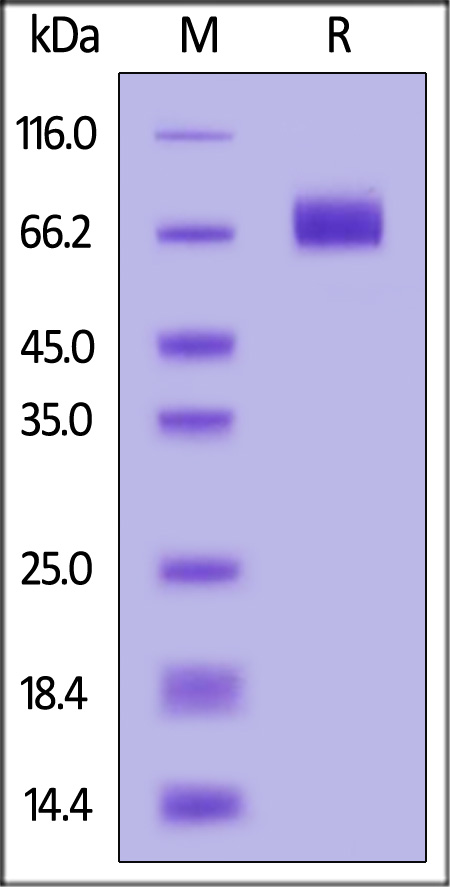
|
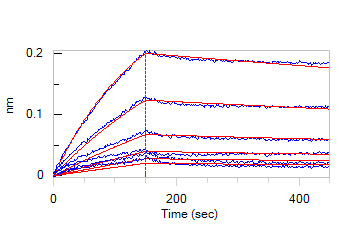
Loaded Mouse IL-10, His Tag (Cat. No. IL0-M4248) on HIS1K Biosensor, can bind Mouse IL-10 R alpha Protein, Fc Tag (Cat. No. ILR-M5254) with an affinity constant of 32.9 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| PEG-ilodecakin | AM-0010 | Phase 3 Clinical | Merck Sharp & Dohme Corp, Armo Biosciences | Solid tumours; Ovarian Neoplasms; Granulomatosis with Polyangiitis; Carcinoma, Renal Cell; Pancreatic Neoplasms; Neoplasms; Vasculitis; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| AG-019 | AG-019 | Phase 2 Clinical | Precigen Actobio Inc | Diabetes Mellitus, Type 1 | Details |
| IBB-0979 | IBB0979; IBB-0979 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| IAE-0972 | IAE-0972 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| Vimekofusp | AMT-101 (Applied Molecular Transport) | Phase 2 Clinical | Applied Molecular Transport | Colitis, Ulcerative | Details |
| Recombinant anti-human EGFR antibody/IL-10 bispecific Fc fusion protein(Dingfu Biotarget) | CmAb-(IL10)2; DF203; DF-203 | Phase 1 Clinical | Dingfu Biotarget Co Ltd | Solid tumours | Details |
| Diakine-DK2 10 (EGFR) | DK210; DK-2-10 | Phase 1 Clinical | Deka Biosciences Inc | Kidney Neoplasms; Solid tumours; Head and Neck Neoplasms; Carcinoma, Renal Cell; Neoplasms; Pancreatic Neoplasms; Skin Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| F8-IL10 | F8-IL10; PF-06687234 | Phase 1 Clinical | Philogen Spa | Inflammatory Bowel Diseases; Arthritis, Rheumatoid; Colitis, Ulcerative | Details |
| PEG-ilodecakin | AM-0010 | Phase 3 Clinical | Merck Sharp & Dohme Corp, Armo Biosciences | Solid tumours; Ovarian Neoplasms; Granulomatosis with Polyangiitis; Carcinoma, Renal Cell; Pancreatic Neoplasms; Neoplasms; Vasculitis; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| AG-019 | AG-019 | Phase 2 Clinical | Precigen Actobio Inc | Diabetes Mellitus, Type 1 | Details |
| IBB-0979 | IBB0979; IBB-0979 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| IAE-0972 | IAE-0972 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| Vimekofusp | AMT-101 (Applied Molecular Transport) | Phase 2 Clinical | Applied Molecular Transport | Colitis, Ulcerative | Details |
| Recombinant anti-human EGFR antibody/IL-10 bispecific Fc fusion protein(Dingfu Biotarget) | CmAb-(IL10)2; DF203; DF-203 | Phase 1 Clinical | Dingfu Biotarget Co Ltd | Solid tumours | Details |
| Diakine-DK2 10 (EGFR) | DK210; DK-2-10 | Phase 1 Clinical | Deka Biosciences Inc | Kidney Neoplasms; Solid tumours; Head and Neck Neoplasms; Carcinoma, Renal Cell; Neoplasms; Pancreatic Neoplasms; Skin Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| F8-IL10 | F8-IL10; PF-06687234 | Phase 1 Clinical | Philogen Spa | Inflammatory Bowel Diseases; Arthritis, Rheumatoid; Colitis, Ulcerative | Details |
This web search service is supported by Google Inc.
