 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
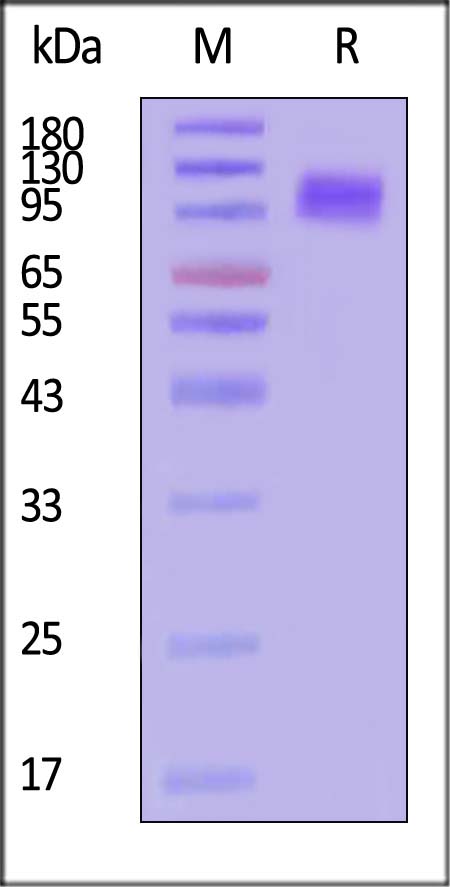
| APP-H52H3 | Human | Human APP Protein, His Tag (active enzyme, MALS verified) |  |
|
|
| CHEK-ATP081 | Human | HEK293/Human APP (GFP) Stable Cell Line | |||
| APP-H51H7 | Human | Human APP / Abeta40 Protein, His Tag |  |

|
|
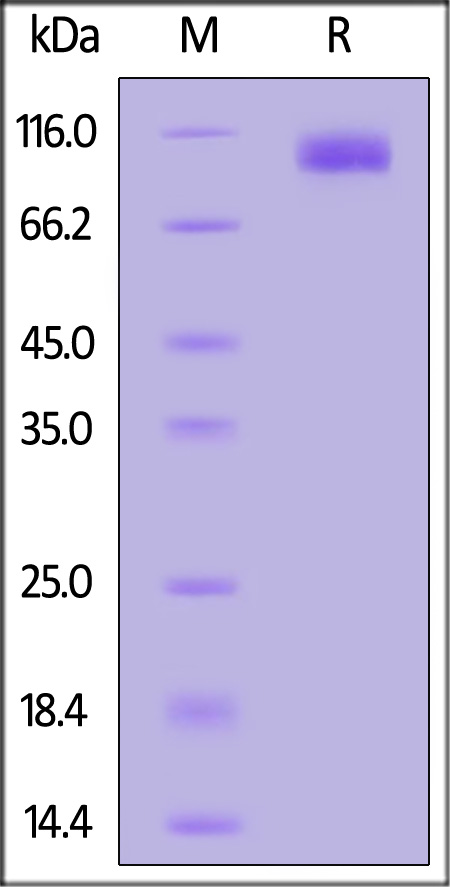
| APP-H52H5 | Human | Human APP / SAPPbeta Protein, His Tag (MALS verified) |  |
|
|
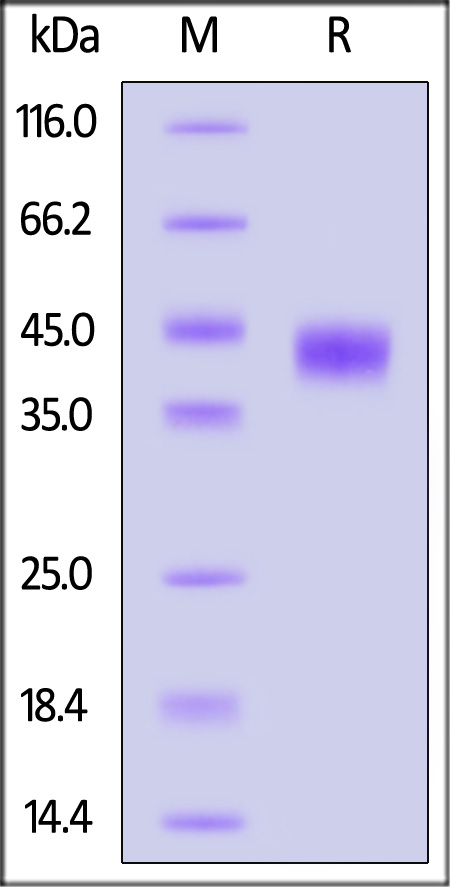
| APP-M52H3 | Mouse | Mouse APP / N-APP Protein, His Tag (MALS verified) |  |
|
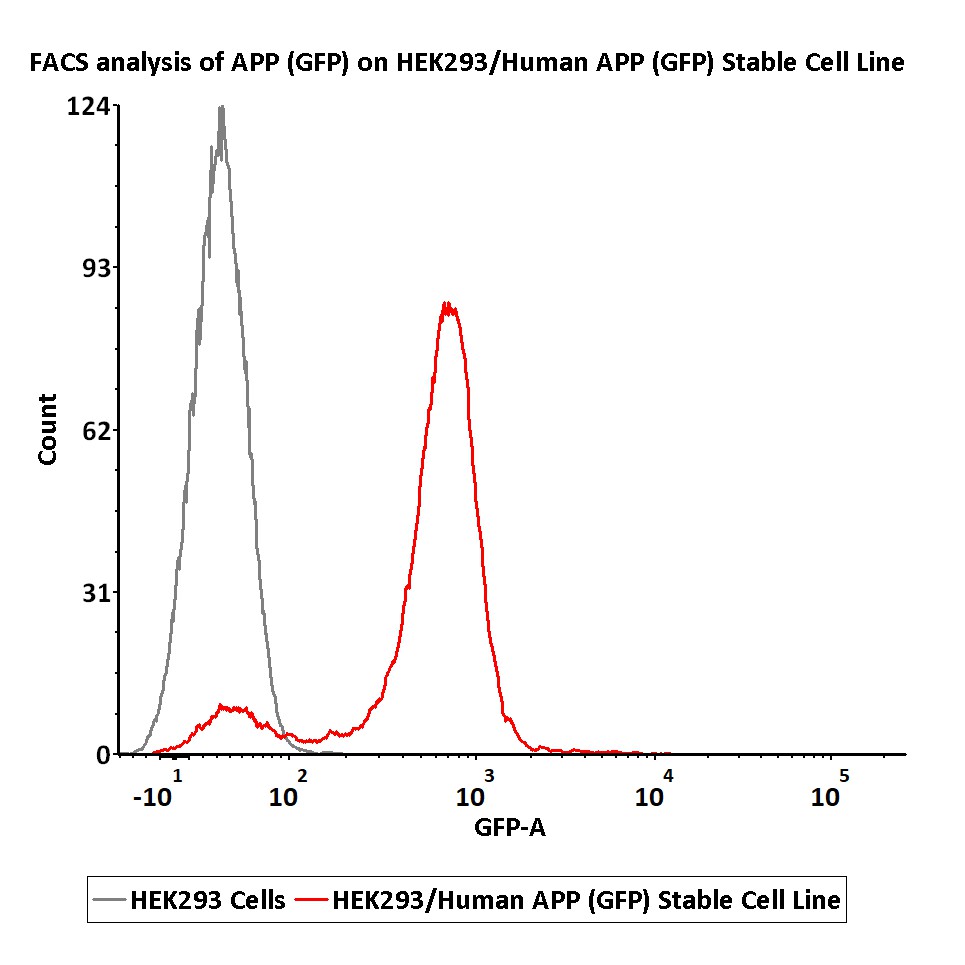
FACS analysis of APP (GFP) on HEK293/Human APP (GFP) Stable Cell Line.
HEK293/Human APP (GFP) Stable Cell Line was red line, negative control HEK293 cells was grey line (QC tested).
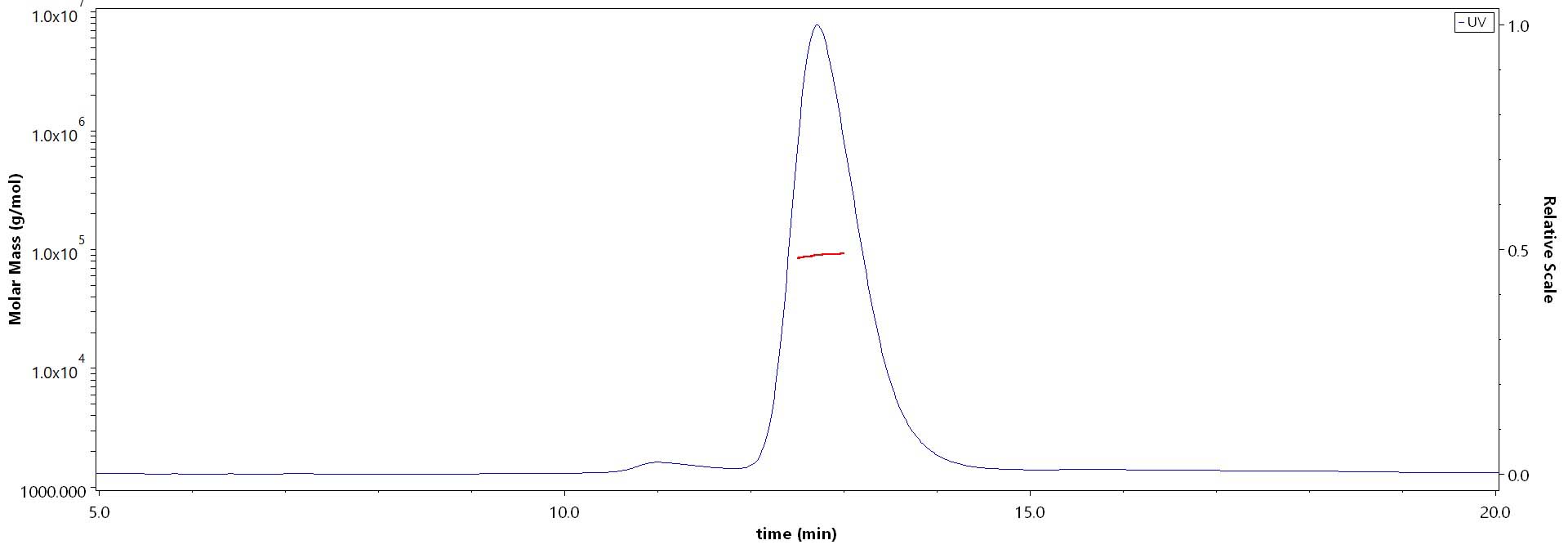
The purity of Human APP Protein, His Tag (Cat. No. APP-H52H3) is more than 85% and the molecular weight of this protein is around 80-110 kDa verified by SEC-MALS.
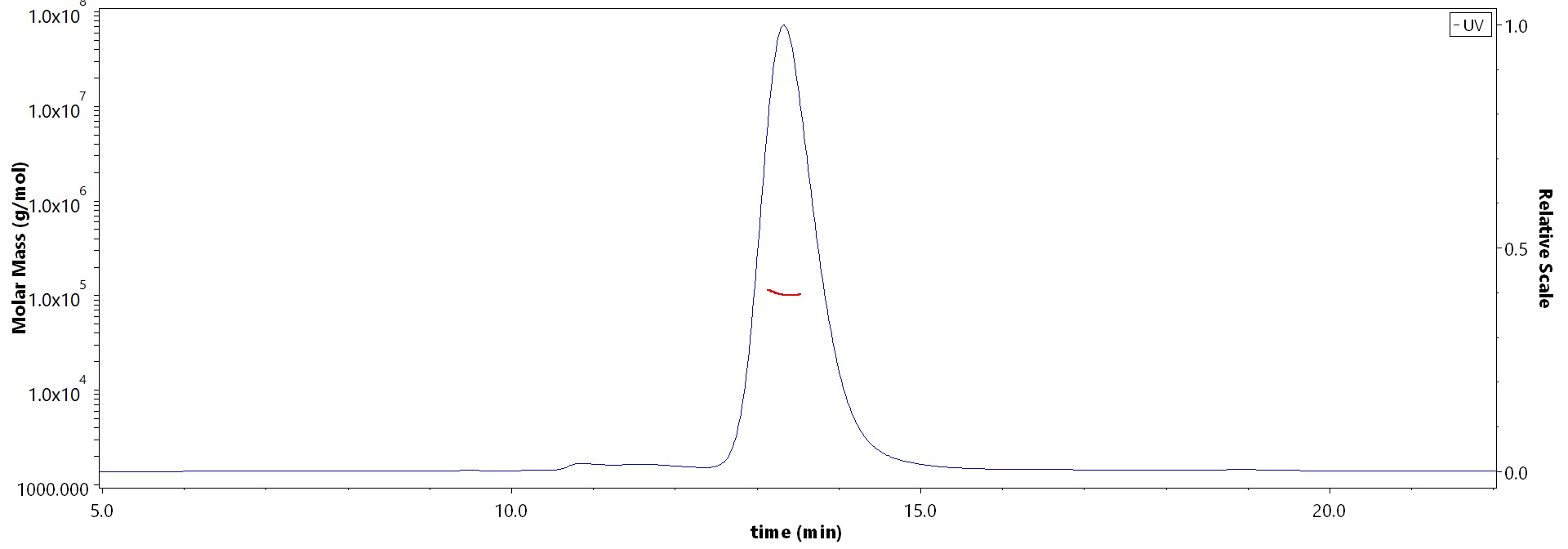
The purity of Human SAPPbeta, His Tag (Cat. No. APP-H52H5) is more than 90% and the molecular weight of this protein is around 95-115 kDa verified by SEC-MALS.
Please contact us via TechSupport@acrobiosystems.com if you have any question on this product.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Sodium Oligomannurarate | GV-971; 971 | Approved | Ocean University Of China, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | 九期一 | Mainland China | Alzheimer Disease | Shanghai Greenvalley Pharmaceutical Co Ltd | 2019-11-02 | Parkinson Disease; Cognitive Dysfunction; Alzheimer Disease | Details |
| Flutemetamol (18F) | AH-110690; GE-067; 18F-GE067 | Approved | Ge Healthcare | Vizamyl | United States | Contrast agents | Ge Healthcare | 2013-10-25 | Atherosclerosis; Cardiomyopathies; Breast Neoplasms; Contrast agents; Cognitive Dysfunction; Alzheimer Disease; Dementia; Diagnostic agents; Hydrocephalus, Normal Pressure; Cognition Disorders | Details |
| Inositol | 4L6452S749 | Approved | Nicosit | Infant, Newborn, Diseases; Precancerous Conditions; Hyperandrogenism; Carcinoma, Non-Small-Cell Lung; Infant, Premature, Diseases; Retinopathy of Prematurity; Lung Neoplasms; Metabolic Diseases; Bronchopulmonary Dysplasia; Anovulation; Diabetic Neuropathies; Insulin Resistance; Glucose Intolerance; Polycystic Ovary Syndrome; Small Cell Lung Carcinoma; Anxiety; Depression | Details | |||||
| Immune globulin 10% (Grifols) | KIg-10; TAL-05-0002 | Approved | Bayer AG | Gamunex, Gammaked | United States | Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Purpura, Thrombocytopenic, Idiopathic; Immunologic Deficiency Syndromes | null | 2003-08-27 | Anxiety Disorders; Corneal Neovascularization; Immunologic Deficiency Syndromes; Kidney Failure, Chronic; Muscular Diseases; Common Variable Immunodeficiency; Neuralgia; Obsessive-Compulsive Disorder; Reflex Sympathetic Dystrophy; Severe Combined Immunodeficiency; Wiskott-Aldrich Syndrome; Autoimmune Diseases; Postural Orthostatic Tachycardia Syndrome; Coronavirus Disease 2019 (COVID-19); Agammaglobulinemia; Tibial Fractures; Primary Immunodeficiency Diseases; Purpura, Thrombocytopenic, Idiopathic; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Myasthenia Gravis | Details |
| Florapronol (18F) | 18F-FC-119S; 18F-FC-119(S) | Approved | Korea Institute Of Radiological And Medical Sciences, Futurechem | Alzavue | Alzheimer Disease | Details | ||||
| Aducanumab | BIIB-037; NI-10; BART | Approved | Neurimmune Ag | ADUHELM | United States | Alzheimer Disease | Biogen Inc | 2021-06-07 | Cognitive Dysfunction; Dementia; Alzheimer Disease | Details |
| L-Lysine hydrochloride/Cyanocobalamin/Inositol | Approved | 兴邦 | Malnutrition | Details | ||||||
| Immune Globulin Subcutaneous (Human) (Octapharma) | Approved | Octapharma | Cutaquig, Octanorm, Gammanorm | United States | Immunologic Deficiency Syndromes | null | 2018-08-02 | Primary Immunodeficiency Diseases; Dermatomyositis; Immunologic Deficiency Syndromes | Details | |
| Florbetaben (18F) | MNI-815; AV-1; ZK-6013443; BAY-949172; 18F-AV-1; 18F-AV1/ZK; AV-1/ZK; BAY 94-9172; UNII-TLA7312TOI | Approved | Piramal | Neuraceq, 欧韦宁 | EU | Alzheimer Disease | Life Radiopharma Berlin Gmbh | 2014-02-20 | Supranuclear Palsy, Progressive; Down Syndrome; Hyponatremia; Immunoglobulin Light-chain Amyloidosis; Contrast agents; Amyloidosis; Alzheimer Disease; Dementia | Details |
| Lecanemab | BAN-2401 | Approved | Bioarctic Neuroscience | Leqembi, 乐意保, LEQEMBI | United States | Alzheimer Disease | Eisai Inc | 2023-01-06 | Alzheimer Disease; Cognitive Dysfunction; Dementia | Details |
| Florbetapir(18F) | MNI-798; AV-45 | Approved | Eli Lilly And Company | Amyvid | United States | Spasms, Infantile; Rheumatic Diseases; Respiration Disorders; Multiple Sclerosis; Eye Diseases; Collagen Diseases; Skin Diseases; Hypersensitivity | Avid Radiopharmaceuticals Inc | 2012-04-06 | Amyloidosis; Hypersensitivity; Frontotemporal Dementia; Neurodegenerative Diseases; Collagen Diseases; Eye Diseases; Lewy Body Disease; Diagnostic agents; Parkinson Disease; Respiration Disorders; Alzheimer Disease; Rheumatic Diseases; Cognitive Dysfunction; Contrast agents; Spasms, Infantile; Skin Diseases; Multiple Sclerosis; Glioblastoma | Details |
| Sodium Oligomannurarate | GV-971; 971 | Approved | Ocean University Of China, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | 九期一 | Mainland China | Alzheimer Disease | Shanghai Greenvalley Pharmaceutical Co Ltd | 2019-11-02 | Parkinson Disease; Cognitive Dysfunction; Alzheimer Disease | Details |
| Flutemetamol (18F) | AH-110690; GE-067; 18F-GE067 | Approved | Ge Healthcare | Vizamyl | United States | Contrast agents | Ge Healthcare | 2013-10-25 | Atherosclerosis; Cardiomyopathies; Breast Neoplasms; Contrast agents; Cognitive Dysfunction; Alzheimer Disease; Dementia; Diagnostic agents; Hydrocephalus, Normal Pressure; Cognition Disorders | Details |
| Inositol | 4L6452S749 | Approved | Nicosit | Infant, Newborn, Diseases; Precancerous Conditions; Hyperandrogenism; Carcinoma, Non-Small-Cell Lung; Infant, Premature, Diseases; Retinopathy of Prematurity; Lung Neoplasms; Metabolic Diseases; Bronchopulmonary Dysplasia; Anovulation; Diabetic Neuropathies; Insulin Resistance; Glucose Intolerance; Polycystic Ovary Syndrome; Small Cell Lung Carcinoma; Anxiety; Depression | Details | |||||
| Immune globulin 10% (Grifols) | KIg-10; TAL-05-0002 | Approved | Bayer AG | Gamunex, Gammaked | United States | Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Purpura, Thrombocytopenic, Idiopathic; Immunologic Deficiency Syndromes | null | 2003-08-27 | Anxiety Disorders; Corneal Neovascularization; Immunologic Deficiency Syndromes; Kidney Failure, Chronic; Muscular Diseases; Common Variable Immunodeficiency; Neuralgia; Obsessive-Compulsive Disorder; Reflex Sympathetic Dystrophy; Severe Combined Immunodeficiency; Wiskott-Aldrich Syndrome; Autoimmune Diseases; Postural Orthostatic Tachycardia Syndrome; Coronavirus Disease 2019 (COVID-19); Agammaglobulinemia; Tibial Fractures; Primary Immunodeficiency Diseases; Purpura, Thrombocytopenic, Idiopathic; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Myasthenia Gravis | Details |
| Florapronol (18F) | 18F-FC-119S; 18F-FC-119(S) | Approved | Korea Institute Of Radiological And Medical Sciences, Futurechem | Alzavue | Alzheimer Disease | Details | ||||
| Aducanumab | BIIB-037; NI-10; BART | Approved | Neurimmune Ag | ADUHELM | United States | Alzheimer Disease | Biogen Inc | 2021-06-07 | Cognitive Dysfunction; Dementia; Alzheimer Disease | Details |
| L-Lysine hydrochloride/Cyanocobalamin/Inositol | Approved | 兴邦 | Malnutrition | Details | ||||||
| Immune Globulin Subcutaneous (Human) (Octapharma) | Approved | Octapharma | Cutaquig, Octanorm, Gammanorm | United States | Immunologic Deficiency Syndromes | null | 2018-08-02 | Primary Immunodeficiency Diseases; Dermatomyositis; Immunologic Deficiency Syndromes | Details | |
| Florbetaben (18F) | MNI-815; AV-1; ZK-6013443; BAY-949172; 18F-AV-1; 18F-AV1/ZK; AV-1/ZK; BAY 94-9172; UNII-TLA7312TOI | Approved | Piramal | Neuraceq, 欧韦宁 | EU | Alzheimer Disease | Life Radiopharma Berlin Gmbh | 2014-02-20 | Supranuclear Palsy, Progressive; Down Syndrome; Hyponatremia; Immunoglobulin Light-chain Amyloidosis; Contrast agents; Amyloidosis; Alzheimer Disease; Dementia | Details |
| Lecanemab | BAN-2401 | Approved | Bioarctic Neuroscience | Leqembi, 乐意保, LEQEMBI | United States | Alzheimer Disease | Eisai Inc | 2023-01-06 | Alzheimer Disease; Cognitive Dysfunction; Dementia | Details |
| Florbetapir(18F) | MNI-798; AV-45 | Approved | Eli Lilly And Company | Amyvid | United States | Spasms, Infantile; Rheumatic Diseases; Respiration Disorders; Multiple Sclerosis; Eye Diseases; Collagen Diseases; Skin Diseases; Hypersensitivity | Avid Radiopharmaceuticals Inc | 2012-04-06 | Amyloidosis; Hypersensitivity; Frontotemporal Dementia; Neurodegenerative Diseases; Collagen Diseases; Eye Diseases; Lewy Body Disease; Diagnostic agents; Parkinson Disease; Respiration Disorders; Alzheimer Disease; Rheumatic Diseases; Cognitive Dysfunction; Contrast agents; Spasms, Infantile; Skin Diseases; Multiple Sclerosis; Glioblastoma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Buntanetap | ANVS-405; ANVS-401 | Phase 3 Clinical | Raptor Pharmaceutical | Down Syndrome; Chronic Traumatic Encephalopathy; Parkinson Disease; Alzheimer Disease; Cognitive Dysfunction; Frontotemporal Dementia | Details |
| Gantenerumab | MAb-31; R-1450; RG-1450; R-04909832 | Phase 3 Clinical | Chugai Pharmaceutical Co Ltd | Alzheimer Disease; Dementia | Details |
| Valiltramiprosate | NRM-8499; ALZ-801; BLU-8499 | Phase 3 Clinical | Alzheon Inc | Alzheimer Disease | Details |
| Solanezumab | LY-2062430 | Phase 3 Clinical | Eli Lilly And Company | Alzheimer Disease; Dementia; Cognition Disorders | Details |
| Birtamimab | NEOD-001 | Phase 3 Clinical | Prothena | Immunoglobulin Light-chain Amyloidosis; Amyloidosis | Details |
| Remternetug | LY-3372993 | Phase 3 Clinical | Eli Lilly And Company | Alzheimer Disease | Details |
| L-clausenamide | Phase 2 Clinical | Guangzhou Nuohao Pharmaceutical Technology Co Ltd, Fuan Pharmaceutical (Group) Co Ltd, Institue Of Materia Medica Chinese Academy Of Medical Science, Qingdao Huanghai Pharmaceutical Co Ltd | Dementia; Cognitive Dysfunction; Memory Disorders | Details | |
| Crenezumab | MABT-5102-A; RG-7412; R-7412; RO-5490245 | Phase 2 Clinical | Ac Immune Sa | Alzheimer Disease | Details |
| 11C-BF-227 | 11C-BF-227; [11C]BF-227 | Phase 2 Clinical | Tohoku University | Diagnostic agents | Details |
| Pittsburgh Compound B | [11C]PIB; [11C]6-OH-BTA-1; 11C-PIB | Phase 2 Clinical | The University Of Utah, University Of Pittsburgh, Ge Healthcare, Uppsala Universitet | Ovarian Neoplasms; Brain Concussion; Breast Neoplasms; Alzheimer Disease; Alcoholism; Dementia; Parkinson Disease | Details |
| CT-1812 | CT-1812; SV-119 | Phase 2 Clinical | Cogrx | Alzheimer Disease; Cognitive Dysfunction; Lewy Body Disease; Macular Degeneration | Details |
| [18F]THK-5105 | [18F]THK-5105 | Phase 2 Clinical | Tohoku University | Alzheimer Disease | Details |
| Edonerpic | T-817 MA | Phase 2 Clinical | Toyama Chemical Co Ltd, Fujifilm Group | Alzheimer Disease; Cognitive Dysfunction; Hepatic Insufficiency | Details |
| ABvac-40 | ABvac-40 | Phase 2 Clinical | Araclon Biotech Sl | Alzheimer Disease; Cognitive Dysfunction | Details |
| PK-051 | PK051; PK-051 | Phase 2 Clinical | PharmaKure Ltd | Alzheimer Disease; Cognitive Dysfunction | Details |
| PRI-002 | RD-2; PRI-002 | Phase 2 Clinical | PRInnovation GmbH, Priavoid | Alzheimer Disease; Cognitive Dysfunction | Details |
| 18F-92 | 18F-92 | Phase 2 Clinical | First Affiliated Hospital Of Fujian Medical University | Alzheimer Disease | Details |
| SHR-1707 | SHR-1707 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| [18F]THK-5117 | [18F]THK-5117 | Phase 2 Clinical | Tohoku University | Alzheimer Disease | Details |
| AD-35 | AD-35 | Phase 2 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| Trontinemab | RO-7126209; RG-6102 | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Alzheimer Disease | Details |
| APH-1105 | APH-1105 | Phase 2 Clinical | Aphios | Dementia; Alzheimer Disease | Details |
| RIV-1061-IR | RIV-1061; RIV-1061-IR | Phase 1 Clinical | Revivo Therapeutics | Alzheimer Disease; Cognition Disorders | Details |
| A ß antibody Fab | Phase 1 Clinical | Eli Lilly And Company | Alzheimer Disease | Details | |
| RQ-9 | AAT-009; RQ-9; RQ-00000009 | Phase 1 Clinical | Pfizer Inc | Dementia | Details |
| ASN-120290 | ASN-561; ASN-90; ASN-120290 | Phase 1 Clinical | Merck Serono | Alzheimer Disease; Parkinson Disease | Details |
| MDR-1339 | DBT-1339; MDR-1339 | Phase 1 Clinical | Medifron | Alzheimer Disease | Details |
| AV-1959D | AV 1959D; AV-1959D | Phase 1 Clinical | Institute For Molecular Medicine, University Of California, Flinders University | Alzheimer Disease | Details |
| ALZ-101 | ALZ-101 | Phase 1 Clinical | Alzinova AB | Alzheimer Disease; Dementia | Details |
| PRX-012 | PRX-012 | Phase 1 Clinical | Prothena | Alzheimer Disease | Details |
| PMN-310 | huPMN-310; PMN-310 | Phase 1 Clinical | ProMIS Neurosciences Inc | Alzheimer Disease; Amyotrophic Lateral Sclerosis; Multiple System Atrophy | Details |
| RP-902 | RP902; RP-902 | Phase 1 Clinical | Risen (Suzhou) Pharma Tech Co Ltd | Alzheimer Disease | Details |
| ACU-193 | ACU-193; ACU193 | Phase 1 Clinical | Acumen Pharmaceuticals Inc | Alzheimer Disease | Details |
| OAB-14 | OAB-14 | Phase 1 Clinical | Shenyang Pharmaceutical University | Alzheimer Disease | Details |
| ALN-APP | Phase 1 Clinical | Sirna Therapeutics Inc | Cerebral Amyloid Angiopathy; Spinocerebellar Degenerations; Alzheimer Disease; Neurodegenerative Diseases | Details | |
| BEY-2153 | BEY-2153 | Phase 1 Clinical | Beyondbio Inc | Alzheimer Disease | Details |
| Abeta42 antibody (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Alzheimer Disease | Details | |
| Anatabine citrate | RCP-006 | Clinical | Rock Creek, Roskamp Institute | Thyroiditis, Autoimmune; Smoking Cessation; Tobacco Use Disorder; Alzheimer Disease; Inflammation | Details |
| 123I-ABC-577 | 123I-ABC-577 | Clinical | Nihon Medi-Physics Co Ltd | Diagnostic agents | Details |
| Tarenflurbil spraygel (MIKA Pharma) | Clinical | Galen Ltd, Mika Pharma Gmbh | Pain; Arthritis, Rheumatoid; Skin Diseases; Inflammation; Dermatitis, Atopic | Details | |
| Procaine Hydrochloride/Vitamin B6/Inositol | Details | ||||
| Inositol/Vitamin B1/Vitamin B2 | Details | ||||
| Buntanetap | ANVS-405; ANVS-401 | Phase 3 Clinical | Raptor Pharmaceutical | Down Syndrome; Chronic Traumatic Encephalopathy; Parkinson Disease; Alzheimer Disease; Cognitive Dysfunction; Frontotemporal Dementia | Details |
| Gantenerumab | MAb-31; R-1450; RG-1450; R-04909832 | Phase 3 Clinical | Chugai Pharmaceutical Co Ltd | Alzheimer Disease; Dementia | Details |
| Valiltramiprosate | NRM-8499; ALZ-801; BLU-8499 | Phase 3 Clinical | Alzheon Inc | Alzheimer Disease | Details |
| Solanezumab | LY-2062430 | Phase 3 Clinical | Eli Lilly And Company | Alzheimer Disease; Dementia; Cognition Disorders | Details |
| Birtamimab | NEOD-001 | Phase 3 Clinical | Prothena | Immunoglobulin Light-chain Amyloidosis; Amyloidosis | Details |
| Remternetug | LY-3372993 | Phase 3 Clinical | Eli Lilly And Company | Alzheimer Disease | Details |
| L-clausenamide | Phase 2 Clinical | Guangzhou Nuohao Pharmaceutical Technology Co Ltd, Fuan Pharmaceutical (Group) Co Ltd, Institue Of Materia Medica Chinese Academy Of Medical Science, Qingdao Huanghai Pharmaceutical Co Ltd | Dementia; Cognitive Dysfunction; Memory Disorders | Details | |
| Crenezumab | MABT-5102-A; RG-7412; R-7412; RO-5490245 | Phase 2 Clinical | Ac Immune Sa | Alzheimer Disease | Details |
| 11C-BF-227 | 11C-BF-227; [11C]BF-227 | Phase 2 Clinical | Tohoku University | Diagnostic agents | Details |
| Pittsburgh Compound B | [11C]PIB; [11C]6-OH-BTA-1; 11C-PIB | Phase 2 Clinical | The University Of Utah, University Of Pittsburgh, Ge Healthcare, Uppsala Universitet | Ovarian Neoplasms; Brain Concussion; Breast Neoplasms; Alzheimer Disease; Alcoholism; Dementia; Parkinson Disease | Details |
| CT-1812 | CT-1812; SV-119 | Phase 2 Clinical | Cogrx | Alzheimer Disease; Cognitive Dysfunction; Lewy Body Disease; Macular Degeneration | Details |
| [18F]THK-5105 | [18F]THK-5105 | Phase 2 Clinical | Tohoku University | Alzheimer Disease | Details |
| Edonerpic | T-817 MA | Phase 2 Clinical | Toyama Chemical Co Ltd, Fujifilm Group | Alzheimer Disease; Cognitive Dysfunction; Hepatic Insufficiency | Details |
| ABvac-40 | ABvac-40 | Phase 2 Clinical | Araclon Biotech Sl | Alzheimer Disease; Cognitive Dysfunction | Details |
| PK-051 | PK051; PK-051 | Phase 2 Clinical | PharmaKure Ltd | Alzheimer Disease; Cognitive Dysfunction | Details |
| PRI-002 | RD-2; PRI-002 | Phase 2 Clinical | PRInnovation GmbH, Priavoid | Alzheimer Disease; Cognitive Dysfunction | Details |
| 18F-92 | 18F-92 | Phase 2 Clinical | First Affiliated Hospital Of Fujian Medical University | Alzheimer Disease | Details |
| SHR-1707 | SHR-1707 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| [18F]THK-5117 | [18F]THK-5117 | Phase 2 Clinical | Tohoku University | Alzheimer Disease | Details |
| AD-35 | AD-35 | Phase 2 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Alzheimer Disease | Details |
| Trontinemab | RO-7126209; RG-6102 | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Alzheimer Disease | Details |
| APH-1105 | APH-1105 | Phase 2 Clinical | Aphios | Dementia; Alzheimer Disease | Details |
| RIV-1061-IR | RIV-1061; RIV-1061-IR | Phase 1 Clinical | Revivo Therapeutics | Alzheimer Disease; Cognition Disorders | Details |
| A ß antibody Fab | Phase 1 Clinical | Eli Lilly And Company | Alzheimer Disease | Details | |
| RQ-9 | AAT-009; RQ-9; RQ-00000009 | Phase 1 Clinical | Pfizer Inc | Dementia | Details |
| ASN-120290 | ASN-561; ASN-90; ASN-120290 | Phase 1 Clinical | Merck Serono | Alzheimer Disease; Parkinson Disease | Details |
| MDR-1339 | DBT-1339; MDR-1339 | Phase 1 Clinical | Medifron | Alzheimer Disease | Details |
| AV-1959D | AV 1959D; AV-1959D | Phase 1 Clinical | Institute For Molecular Medicine, University Of California, Flinders University | Alzheimer Disease | Details |
| ALZ-101 | ALZ-101 | Phase 1 Clinical | Alzinova AB | Alzheimer Disease; Dementia | Details |
| PRX-012 | PRX-012 | Phase 1 Clinical | Prothena | Alzheimer Disease | Details |
| PMN-310 | huPMN-310; PMN-310 | Phase 1 Clinical | ProMIS Neurosciences Inc | Alzheimer Disease; Amyotrophic Lateral Sclerosis; Multiple System Atrophy | Details |
| RP-902 | RP902; RP-902 | Phase 1 Clinical | Risen (Suzhou) Pharma Tech Co Ltd | Alzheimer Disease | Details |
| ACU-193 | ACU-193; ACU193 | Phase 1 Clinical | Acumen Pharmaceuticals Inc | Alzheimer Disease | Details |
| OAB-14 | OAB-14 | Phase 1 Clinical | Shenyang Pharmaceutical University | Alzheimer Disease | Details |
| ALN-APP | Phase 1 Clinical | Sirna Therapeutics Inc | Cerebral Amyloid Angiopathy; Spinocerebellar Degenerations; Alzheimer Disease; Neurodegenerative Diseases | Details | |
| BEY-2153 | BEY-2153 | Phase 1 Clinical | Beyondbio Inc | Alzheimer Disease | Details |
| Abeta42 antibody (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Alzheimer Disease | Details | |
| Anatabine citrate | RCP-006 | Clinical | Rock Creek, Roskamp Institute | Thyroiditis, Autoimmune; Smoking Cessation; Tobacco Use Disorder; Alzheimer Disease; Inflammation | Details |
| 123I-ABC-577 | 123I-ABC-577 | Clinical | Nihon Medi-Physics Co Ltd | Diagnostic agents | Details |
| Tarenflurbil spraygel (MIKA Pharma) | Clinical | Galen Ltd, Mika Pharma Gmbh | Pain; Arthritis, Rheumatoid; Skin Diseases; Inflammation; Dermatitis, Atopic | Details | |
| Procaine Hydrochloride/Vitamin B6/Inositol | Details | ||||
| Inositol/Vitamin B1/Vitamin B2 | Details |
This web search service is supported by Google Inc.
