 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
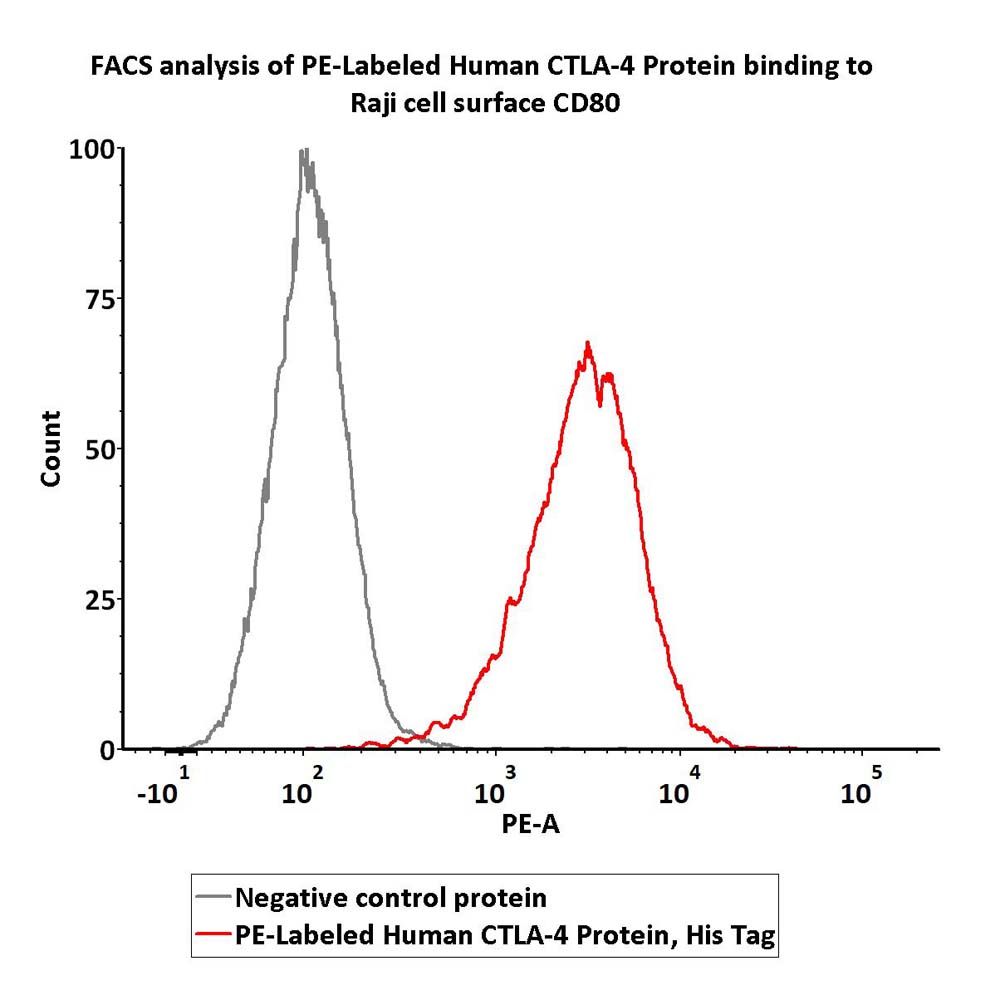
Flow cytometric analysis of Raji cells staining with PE-Labeled Human CTLA-4 Protein, His Tag (Cat. No. CT4-HP2H3) at 1:50 dilution (2μL of the antibody stock solution corresponds to labeling of 5e5 cells in a final volume of 100 µL) , compared with negative control protein. PE signal was used to evaluate the binding activity(QC tested).
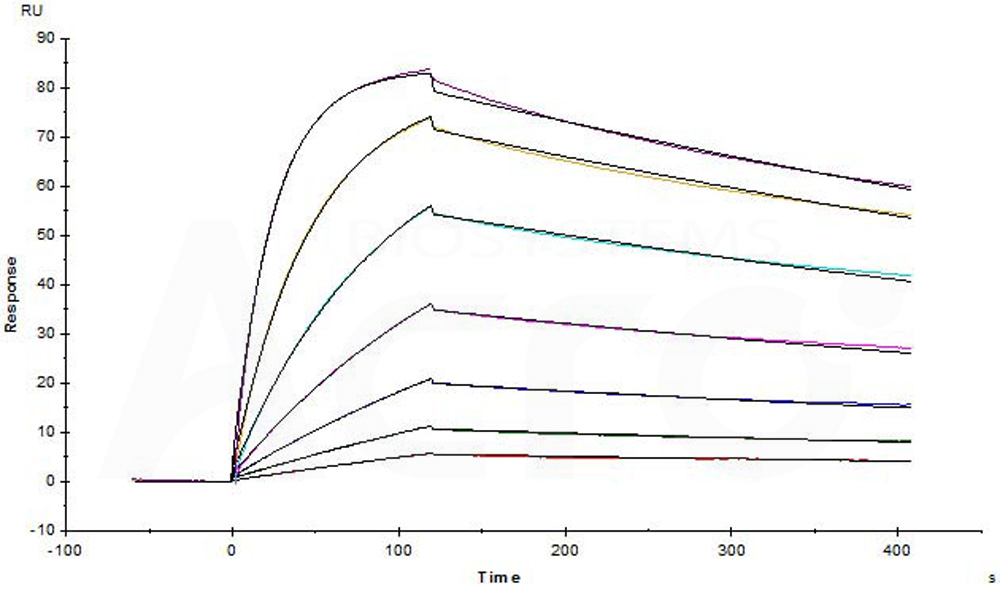
Biotinylated Human CTLA-4, His,Avitag (Cat. No. CT4-H82E3) captured on Biotin CAP - Series S sensor Chip can bind Yervoy (Ipilimumab) with an affinity constant of 0.635 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Cadonilimab | AK-104; AK104 | Approved | Zhongshan Akeso Biopharma Co Ltd | 开坦尼 | Mainland China | Uterine Cervical Neoplasms | Kangfang Pharmaceutical Co Ltd | 2022-06-29 | Nasopharyngeal Carcinoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Uterine Cervical Neoplasms; Esophageal Squamous Cell Carcinoma; DNA Repair-Deficiency Disorders; Urologic Neoplasms; Colorectal Neoplasms; Vulvar Neoplasms; Microsatellite Instability; Solid tumours; Microsatellite instability-high cancer; Triple Negative Breast Neoplasms; Neoplasms; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Vaginal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Lymphoma, T-Cell, Peripheral | Details |
| Ipilimumab | 10D1; MDX-CTLA-4; Anti-CTLA-4 Mab; BMS-734016; Mab-10D14; MDX-101; MDX-010 | Approved | Bristol-Myers Squibb Company | Yervoy, 逸沃 | United States | Melanoma | Bristol-Myers Squibb Company | 2011-03-25 | Primary Myelofibrosis; Brain metastases; Neoplasms, Gonadal Tissue; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Burkitt Lymphoma; Testicular Neoplasms; Waldenstrom Macroglobulinemia; Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative; Colorectal Neoplasms; Sezary Syndrome; Sarcoma; Neuroblastoma; Breast Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Neoplasm Metastasis; Neoplasms, Germ Cell and Embryonal; Uterine Cervical Neoplasms; Endodermal Sinus Tumor; Leukemia, T-Cell; Leukemia, Large Granular Lymphocytic; Adenocarcinoma; Leukemia, Lymphocytic, Chronic, B-Cell; Mycosis Fungoides; Carcinoma, Hepatocellular; Prostatic Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Embryonal; Choriocarcinoma; Carcinoma, Squamous Cell; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Intraocular Lymphoma; Lymphoma, T-Cell; Squamous Cell Carcinoma of Head and Neck; Carc | Details |
| Tremelimumab | CP-675206; CP-675 | Approved | Pfizer Inc | IMJUDO | United States | Carcinoma, Hepatocellular | Astrazeneca Ab | 2022-10-21 | Urogenital Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Vulvar Neoplasms; Urethral Neoplasms; Colorectal Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Oropharyngeal Neoplasms; Ureteral Neoplasms; Peritoneal Neoplasms; Genital Neoplasms, Female; Carcinoma, Squamous Cell; Mouth Neoplasms; Prostatic Neoplasms; Gallbladder Neoplasms; Esophageal adenocarcinoma; Glioma; Lung Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung; Hepatitis C, Chronic; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Endometrioid; Cystadenoma, Serous; Solid tumours; Head and Neck Neoplasms; Hematologic Neoplasms; Bone metastases; Kidney Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms; HIV Infections; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Vaginal Neoplasms; Carcinoma; Liver Neoplasms; Cystadenocarcinoma, Serous; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Neopl | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| CS-1002 | SHR-8068; CS-1002 | Phase 3 Clinical | Cstone Pharmaceuticals | Solid tumours; Stomach Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Botensilimab | AGEN-1181 | Phase 3 Clinical | Agenus Inc | Colonic Neoplasms; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Adenocarcinoma; Carcinoma, Pancreatic Ductal; Endometrial Neoplasms; Esophageal adenocarcinoma; Colorectal Neoplasms; Prostatic Neoplasms; Microsatellite Instability; Solid tumours; Pancreatic Neoplasms; Neoplasms; Fibrolamellar hepatocellular carcinoma; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Stomach Neoplasms; Esophageal Neoplasms; Ovarian Neoplasms; Liver Neoplasms | Details |
| Gotistobart | ONC-392; BNT316; BNT-316 | Phase 3 Clinical | Oncoimmune Inc | Carcinoma, Adenoid Cystic; Uterine Cervical Neoplasms; Adenocarcinoma; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Ovarian Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Salivary Gland Neoplasms; Pancreatic Neoplasms; Cystadenocarcinoma, Serous; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Anorectal disorders; Solid tumours; Head and Neck Neoplasms | Details |
| Erfonrilimab | KN046; KN-046 | Phase 3 Clinical | Jiangsu Alphamab Biopharmaceuticals Co Ltd | Colorectal Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Thymoma; Esophageal Squamous Cell Carcinoma; Lymphoma; Solid tumours; Thymus Neoplasms; Breast Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Biliary Tract Neoplasms | Details |
| Volrustomig | MEDI-5752; MEDI5752 | Phase 3 Clinical | Medimmune Llc | Biliary Tract Neoplasms; Solid tumours; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoma, Renal Cell; Mesothelioma; Sarcoma; Bile Duct Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| BCD-217 | BCD-217 | Phase 3 Clinical | Biocad | Melanoma | Details |
| Ipilimumab biosimilar (Innovent Biologics) | IBI-310 | Phase 3 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Biliary Tract Neoplasms; Liver Neoplasms; Esophageal Neoplasms; Colonic Neoplasms; Neuroendocrine Tumors; Nasopharyngeal Carcinoma; Microsatellite Instability; Colorectal Neoplasms; Bile Duct Neoplasms; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Pembrolizumab/Quavonlimab | MK-1308A | Phase 3 Clinical | Merck Sharp & Dohme Corp, Msd Ireland (Carlow), MSD R&D(China)Co Ltd | Solid tumours; Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular; Melanoma | Details |
| YH-001 | YH-001 | Phase 2 Clinical | Eucure Pharmaceutical Technology (Beijing) Co Ltd | Liver Neoplasms; Solid tumours; Alopecia Areata; Sarcoma; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| SI-B003 | SI-B003 | Phase 2 Clinical | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Breast Neoplasms; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Neoplasm Metastasis; Melanoma | Details | |
| BMS-986288 | BMS-986288 | Phase 2 Clinical | Cytomx Therapeutics Inc | Neoplasms | Details |
| Evalstotug | CAB-CTLA-4; BA-3071 | Phase 2 Clinical | Bioatla, Beigene Ltd | Solid tumours; Carcinoma, Renal Cell; Stomach Neoplasms; Carcinoma; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Vudalimab | XmAb-20717; XmAb-717; XmAb 717 | Phase 2 Clinical | Xencor Inc | Thymoma; Adenocarcinoma, Clear Cell; Cholangiocarcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Vulvar Neoplasms; Prostatic Neoplasms; Astrocytoma; Colorectal Neoplasms; Endometrial Neoplasms; Microsatellite instability-high cancer; Carcinoma, Neuroendocrine; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Thyroid Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Ovarian Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Carcinoma, Basal Cell; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Biliary Tract Neoplasms; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Hodgkin Disease; Adenoma, Acidophil; Colonic Neoplasms; Mesothelioma; Adnexal Diseases; Prostatic Neoplasms, Castration-Resistant | Details |
| Recombinant anti-CTLA-4 human monoclonal antibody (Hualan Biological Engineering) | Phase 2 Clinical | Hualan Genetic Engineering Co Ltd | Melanoma | Details | |
| Lorigerlimab | MGD-019; AEX1344 | Phase 2 Clinical | Macrogenics Inc | Solid tumours; Liver Neoplasms; Skin Melanoma; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Pancreatic Ductal; Uterine Cervical Neoplasms; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| PD-L1/CTLA4 bispecific monoclonal antibody (Changhai Hospital) | Phase 2 Clinical | Changhai Hospital Of Shanghai | Pancreatic Neoplasms | Details | |
| RP-2 | RP-2 | Phase 2 Clinical | Replimune Group Inc, Bristol-Myers Squibb Company | Neoplasms; Colorectal Neoplasms | Details |
| Abatacept (Orban Biotech) | Phase 2 Clinical | Orban Biotech, The National Institute Of Diabetes And Digestive And Kidney Diseases | Diabetes Mellitus, Type 1 | Details | |
| Quavonlimab | MK-1308; AK-107 | Phase 2 Clinical | Zhongshan Akeso Biopharma Co Ltd, Merck Sharp & Dohme Corp | Solid tumours; Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Zalifrelimab | AGEN-1884; RebmAb-600; UGN-301 | Phase 2 Clinical | 4-Antibody, Ludwig Institute For Cancer Research | Solid tumours; Carcinoma; Hemangiosarcoma; Urinary Bladder Neoplasms; Sarcoma; Urologic Neoplasms; Carcinoma, Pancreatic Ductal; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BMS-986218 (Bristol-Myers Squibb) | BMS-986218 | Phase 2 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Adrenal Gland Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Lung Neoplasms | Details |
| Anti-CTLA-4 and PD-1 CAR-T cell therapy (Shanghai Cell Therapy Research Institute) | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Neoplasms | Details | |
| KD-6001 | KD-6001 | Phase 2 Clinical | Shanghai Kanda Bio-Technology Co Ltd, Shanghai Celgen Bio-Pharmaceutical Co Ltd | Liver Neoplasms; Solid tumours; Neoplasms; Melanoma; Carcinoma, Hepatocellular | Details |
| BT-001 | BT-001 | Phase 2 Clinical | Transgene Sa | Carcinoma, Merkel Cell; Triple Negative Breast Neoplasms; Neoplasms; Sarcoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Porustobart | HBM-4003; HCAb 4003-2 | Phase 2 Clinical | Harbour Biomed | Solid tumours; Neoplasms; Neuroendocrine Tumors; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| JK-08 | JK-08 | Phase 2 Clinical | Xinlitai (Chengdu) Biological Technology Co Ltd | Small Cell Lung Carcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Melanoma; Carcinoma, Squamous Cell; Thyroid Neoplasms; Colorectal Neoplasms; Carcinoma, Ovarian Epithelial; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms | Details |
| Sovipostobart | BMS-986249 | Phase 2 Clinical | Cytomx Therapeutics Inc | Neoplasms | Details |
| GI-101 | SIM-323; SIM323; GI-101 | Phase 2 Clinical | GI Innovation Inc | Solid tumours; Carcinoma, Merkel Cell; Carcinoma, Renal Cell; Vaginal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Vulvar Neoplasms; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Neoplasm Metastasis | Details |
| Firastotug | ADG-116 | Phase 2 Clinical | Adagene (Suzhou) Ltd | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| COYA-302 | COYA302; COYA 302; COYA-302 | Phase 2 Clinical | Coya Therapeutics Inc | Parkinson Disease; Frontotemporal Dementia; Amyotrophic Lateral Sclerosis | Details |
| GI-102 | GI-102 | Phase 2 Clinical | GI Innovation Inc | Solid tumours; Neoplasm Metastasis | Details |
| Anti-CTLA-4/PD-1 expressing EGFR-CAR-T | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Solid tumours | Details | |
| XTX-101 | XTX-101 | Phase 2 Clinical | Xilio Development Inc | Solid tumours | Details |
| RP-3 | RP-3 | Phase 2 Clinical | Replimune Inc | Solid tumours; Liver Neoplasms; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| Muzastotug | ADG-126 | Phase 2 Clinical | Adagene Inc | Liver Neoplasms; Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| PD-L1 antibody combined with CTLA-4 antibody (Shanghai Zhongshan Hospital) | Phase 2 Clinical | Shanghai Zhongshan Hospital | Cholangiocarcinoma | Details | |
| JS-007 | JS007; JS-007 | Phase 2 Clinical | Shanghai Junshi Biosciences Co Ltd | Solid tumours; Pancreatic Neoplasms; Neoplasms; Lung Neoplasms; Melanoma | Details |
| KN-044 | KN-044 | Phase 1 Clinical | Suzhou Alphamab Co Ltd | Solid tumours | Details |
| Bavunalimab | XmAb-22841; XmAb-841 | Phase 1 Clinical | Xencor Inc | Cholangiocarcinoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Fallopian Tube Neoplasms; Penile Neoplasms; Endometrial Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Nasopharyngeal Carcinoma; Solid tumours; Prostatic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck | Details |
| ATOR-1015 | ADC-1015; ATOR-1015 | Phase 1 Clinical | Alligator Bioscience Ab | Solid tumours; Neoplasms | Details |
| Anti-CTLA-4 monoclonal antibody (Regeneron) | REGN-4659 | Phase 1 Clinical | Carcinoma, Non-Small-Cell Lung | Details | |
| Ipilimumab biosimilar (Henlius) | HLX13 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Liver Neoplasms; Solid tumours; Esophageal Neoplasms; Carcinoma, Renal Cell; Mesothelioma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Melanoma | Details |
| Ipilimumab biosimilar (Mab-Venture/ShuangLu Pharmaceutical) | MV-049 | Phase 1 Clinical | Beijing Sl Pharmaceutical Co Ltd, Mab-Venture Biopharm Co Ltd | Solid tumours | Details |
| SAL-008 | SAL-008 | Phase 1 Clinical | Xinlitai (Chengdu) Biological Technology Co Ltd | Neoplasms | Details |
| GIGA-564 | GIGA-564 | Phase 1 Clinical | GigaGen Inc | Solid tumours; Neoplasms | Details |
| A-337 (Klus Pharma) | A337 (Klus Pharma); A-337 (Klus Pharma) | Phase 1 Clinical | Klus Pharma Inc | Neoplasms | Details |
| BZE-2209 | BZE-2209; BZE2209; αPD1/CTLA4-MSLN-CAR T Cells | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Solid tumours | Details |
| MT-8421 | MT-8421; CTLA-4 ETB | Phase 1 Clinical | Molecular Templates Inc | Squamous Cell Carcinoma of Head and Neck; Mismatch Repair Deficient Cancer; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Microsatellite instability-high cancer; Mesothelioma; Esophageal Squamous Cell Carcinoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular | Details |
| STW204/Gotistobart | AI-061 | Phase 1 Clinical | Oncoc4 Inc | Colorectal Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Fallopian Tube Neoplasms; Bile Duct Neoplasms; Carcinoma, Renal Cell; Peritoneal Neoplasms; Digestive System Neoplasms; Urinary Bladder Neoplasms; Stomach Neoplasms; Anus Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Cystadenocarcinoma, Serous | Details |
| KM602 | KM602; KM-602; XZP-KM602 | Phase 1 Clinical | Xuanzhu (Shijiazhuang) Biotechnology Co Ltd | Solid tumours | Details |
| TG-6050 | TG-6050 | Phase 1 Clinical | Transgene Sa | Carcinoma, Non-Small-Cell Lung | Details |
| IMM-27M | IMM27M; IMM-27M | Phase 1 Clinical | ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd | Solid tumours; Neoplasms; Pathologic Processes | Details |
| TWP-102 | TWP-102 | Phase 1 Clinical | Therawisdom Biopharma Co Ltd | Neoplasms | Details |
| SKB-337 | SKB337; A-337; SKB-337 | Phase 1 Clinical | Sichuan Kelun-Biotech Biopharmaceutical Co Ltd | Solid tumours | Details |
| CD-200-AR-L | CD-200-AR-L; hP-1-A-8 | Phase 1 Clinical | University Of Minnesota, OX2 Therapeutics | Glioblastoma; Glioma | Details |
| BAT-4706 | BAT-4706 | Phase 1 Clinical | Bio-Thera Solutions Ltd | Solid tumours; Melanoma | Details |
| FPT-155 | CD80-Fc; FPT-155 | Phase 1 Clinical | Five Prime Therapeutics Inc | Solid tumours | Details |
| B7-2/GM-CSF cancer gene therapy | CIT | Phase 1 Clinical | Radient | Neoplasms | Details |
This web search service is supported by Google Inc.
