 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CXR-H5222 | Human | Human CXADR / CAR Protein, His Tag |  |
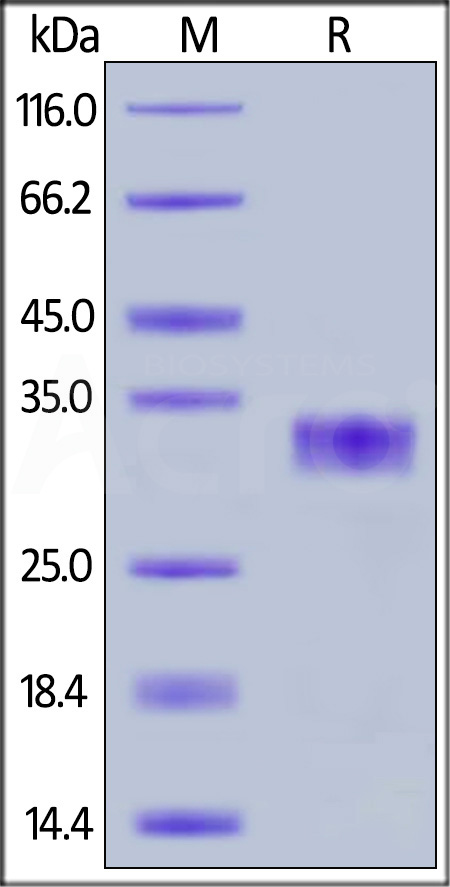
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Upacicalcet | SK-1403; AJT-240 | Approved | Ajinomoto Co Inc | ウパシタ, Upasita | Japan | Hyperparathyroidism, Secondary | Sanwa Kagaku Kenkyusho Co Ltd | 2021-06-23 | Hyperparathyroidism, Secondary | Details |
| Parathyroid hormone (Shire) | NPSP-795; rhPTH (1-84); rhPTH-1-84; PTH (1-84); SB-423562; PTH 1-84; NPSP-558; SHP-635; SHP-634; ALX-111; 423562; EBP-05; EB-612 | Approved | Shire | Natpara, Preotact, Natpar | United States | Hypoparathyroidism | Nps Pharmaceuticals, Inc | 2006-04-24 | Osteoporosis; Bone Diseases, Endocrine; Osteoporosis, Postmenopausal; Hypocalcemia; Psoriasis; Hypoparathyroidism; Hyperparathyroidism | Details |
| Cinacalcet Hydrochloride | AMG-073.HCl; NPS-1493; AMG-073; KRN-1493 | Approved | Amgen Inc | Parareg, Sensipar, Mimpara, Regpara, 盖平, Sensipar/Mimpara | United States | Hypercalcemia; Hyperparathyroidism, Secondary | Amgen Inc | 2004-03-08 | Hyperparathyroidism, Secondary; Rejection of renal transplantation; Hypophosphatemia; Edema, Cardiac; Parathyroid Neoplasms; Nephrosis; Kidney Diseases; Hypercalcemia; Hyperparathyroidism, Primary; Prostatic Neoplasms; Osteomalacia; Renal Insufficiency, Chronic; Hyperparathyroidism; Kidney Failure, Chronic; Chronic Kidney Disease-Mineral and Bone Disorder | Details |
| Etelcalcetide Hydrochloride | ONO-5163; KAI-4169; AMG-416; KAI-4169-HCl | Approved | Amgen Inc | Parsabiv | EU | Hyperparathyroidism, Secondary | Amgen Europe Bv | 2016-11-11 | Hyperparathyroidism, Secondary; Edema, Cardiac; Renal Insufficiency, Chronic; Kidney Failure, Chronic | Details |
| Strontium Ranelate | S-12911; S-12911-2; FK-481 | Approved | Laboratoires Servier | Osseor, Protos, Protelos, 欧思美 | Mainland China | Osteoporosis, Postmenopausal | Les Laboratoires Servier | 2004-09-20 | Osteoporosis, Postmenopausal | Details |
| Evocalcet | KHK-7580; MT-4580 | Approved | Mitsubishi Tanabe Pharma Corp | Orkedia | Japan | Hyperparathyroidism, Secondary | Kyowa Hakko Kirin Co Ltd | 2018-03-23 | Hyperparathyroidism, Secondary; Parathyroid Neoplasms; Hyperparathyroidism, Primary; Hyperparathyroidism | Details |
| Upacicalcet | SK-1403; AJT-240 | Approved | Ajinomoto Co Inc | ウパシタ, Upasita | Japan | Hyperparathyroidism, Secondary | Sanwa Kagaku Kenkyusho Co Ltd | 2021-06-23 | Hyperparathyroidism, Secondary | Details |
| Parathyroid hormone (Shire) | NPSP-795; rhPTH (1-84); rhPTH-1-84; PTH (1-84); SB-423562; PTH 1-84; NPSP-558; SHP-635; SHP-634; ALX-111; 423562; EBP-05; EB-612 | Approved | Shire | Natpara, Preotact, Natpar | United States | Hypoparathyroidism | Nps Pharmaceuticals, Inc | 2006-04-24 | Osteoporosis; Bone Diseases, Endocrine; Osteoporosis, Postmenopausal; Hypocalcemia; Psoriasis; Hypoparathyroidism; Hyperparathyroidism | Details |
| Cinacalcet Hydrochloride | AMG-073.HCl; NPS-1493; AMG-073; KRN-1493 | Approved | Amgen Inc | Parareg, Sensipar, Mimpara, Regpara, 盖平, Sensipar/Mimpara | United States | Hypercalcemia; Hyperparathyroidism, Secondary | Amgen Inc | 2004-03-08 | Hyperparathyroidism, Secondary; Rejection of renal transplantation; Hypophosphatemia; Edema, Cardiac; Parathyroid Neoplasms; Nephrosis; Kidney Diseases; Hypercalcemia; Hyperparathyroidism, Primary; Prostatic Neoplasms; Osteomalacia; Renal Insufficiency, Chronic; Hyperparathyroidism; Kidney Failure, Chronic; Chronic Kidney Disease-Mineral and Bone Disorder | Details |
| Etelcalcetide Hydrochloride | ONO-5163; KAI-4169; AMG-416; KAI-4169-HCl | Approved | Amgen Inc | Parsabiv | EU | Hyperparathyroidism, Secondary | Amgen Europe Bv | 2016-11-11 | Hyperparathyroidism, Secondary; Edema, Cardiac; Renal Insufficiency, Chronic; Kidney Failure, Chronic | Details |
| Strontium Ranelate | S-12911; S-12911-2; FK-481 | Approved | Laboratoires Servier | Osseor, Protos, Protelos, 欧思美 | Mainland China | Osteoporosis, Postmenopausal | Les Laboratoires Servier | 2004-09-20 | Osteoporosis, Postmenopausal | Details |
| Evocalcet | KHK-7580; MT-4580 | Approved | Mitsubishi Tanabe Pharma Corp | Orkedia | Japan | Hyperparathyroidism, Secondary | Kyowa Hakko Kirin Co Ltd | 2018-03-23 | Hyperparathyroidism, Secondary; Parathyroid Neoplasms; Hyperparathyroidism, Primary; Hyperparathyroidism | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Encaleret sulfate | MK-5442; JTT-305; CLTX-305 | Phase 3 Clinical | Japan Tobacco (Hong Kong) Ltd | Osteoporosis; Hypocalcemia; Osteoporosis, Postmenopausal; Hypoparathyroidism | Details |
| LNP-1892 | LNP-1892 | Phase 2 Clinical | Lupin Ltd | Hyperparathyroidism | Details |
| DS-9194b | DS-9194b | Phase 1 Clinical | Daiichi Sankyo Co Ltd | Osteoporosis | Details |
| OSM-0205 | OSM-0205 | Phase 1 Clinical | Yale University, Osmol Therapeutics Inc | Peripheral Nervous System Diseases; Peripheral Nerve Injuries | Details |
| RT-102(Rani) | RT-102 | Phase 1 Clinical | Rani Therapeutics Holdings Inc | Osteoporosis | Details |
| Encaleret sulfate | MK-5442; JTT-305; CLTX-305 | Phase 3 Clinical | Japan Tobacco (Hong Kong) Ltd | Osteoporosis; Hypocalcemia; Osteoporosis, Postmenopausal; Hypoparathyroidism | Details |
| LNP-1892 | LNP-1892 | Phase 2 Clinical | Lupin Ltd | Hyperparathyroidism | Details |
| DS-9194b | DS-9194b | Phase 1 Clinical | Daiichi Sankyo Co Ltd | Osteoporosis | Details |
| OSM-0205 | OSM-0205 | Phase 1 Clinical | Yale University, Osmol Therapeutics Inc | Peripheral Nervous System Diseases; Peripheral Nerve Injuries | Details |
| RT-102(Rani) | RT-102 | Phase 1 Clinical | Rani Therapeutics Holdings Inc | Osteoporosis | Details |
This web search service is supported by Google Inc.
