 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
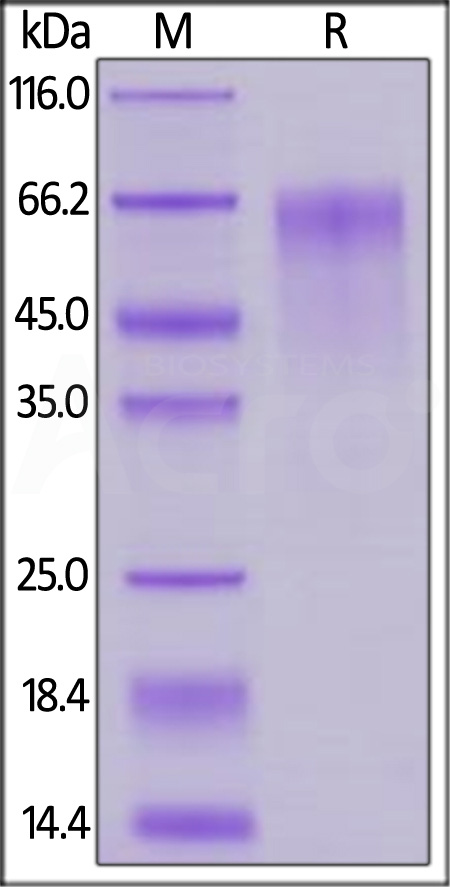
| FG4-H5253 | Human | Human FGF R4 / CD334 Protein, Fc Tag (MALS & SPR verified) |  |
|
|
| FG4-M52Ha | Mouse | Mouse FGF R4 / CD334 Protein, His Tag | 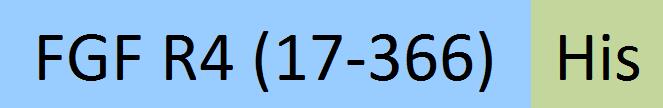 |
|
|
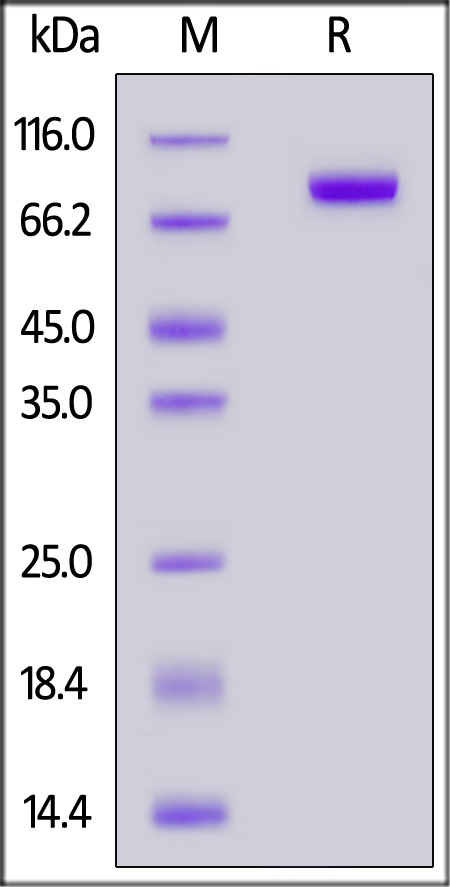
| FG4-H5228 | Human | Human FGF R4 / CD334 Protein, His Tag |  |
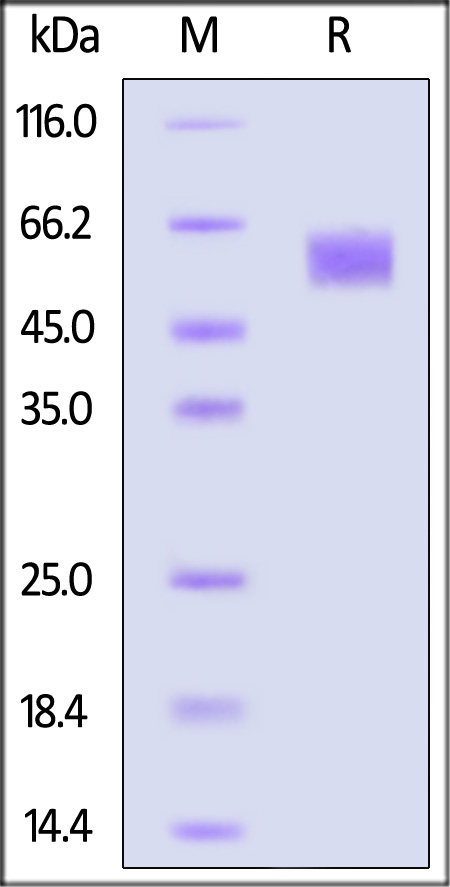
|
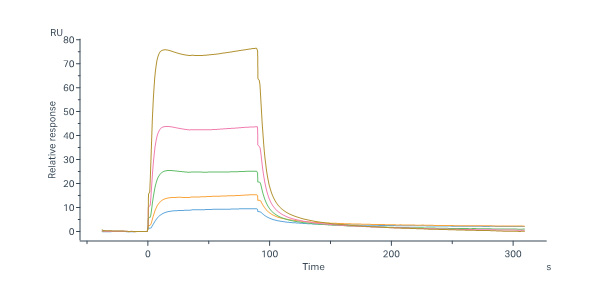
Human FGF R4, Fc Tag (Cat. No. FG4-H5253) immobilized on CM5 Chip can bind Human FGF-4 Protein, His Tag (Cat. No. FG4-H51H3) with an affinity constant of 1.48 μM as determined in a SPR assay (Biacore 8K) (QC tested).
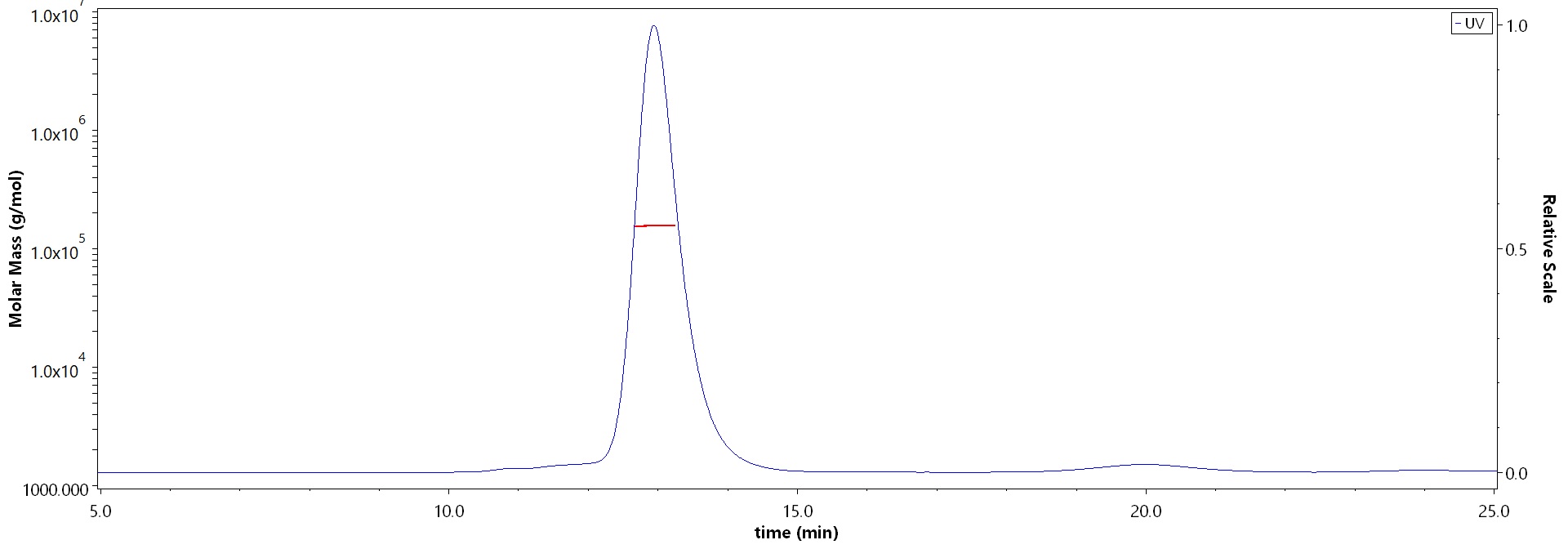
The purity of Human FGF R4, Fc Tag (Cat. No. FG4-H5253) is more than 90% and the molecular weight of this protein is around 145-175 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Biliary Tract Neoplasms; Solid tumours; Bone Marrow Neoplasms; Carcinoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Central Nervous System Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Sarcoma; Endometrial Neoplasms; Lymphoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Erdafitinib | G-024; JNJ-493; JNJ-42756493; TAR-210; 890E37NHMV | Approved | Astex Pharmaceuticals Inc | Balversa | United States | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Carcinoma, Squamous Cell; Breast Neoplasms; Neuroblastoma; Sarcoma; Prostatic Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Histiocytic Sarcoma; Hepatic Insufficiency; Lymphoma; Lymphoma, Non-Hodgkin; Histiocytosis, Langerhans-Cell; Carcinoma, Neuroendocrine; Neoplasms, Neuroepithelial; Glioma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Xanthogranuloma, Juvenile; Neuroectodermal Tumors, Primitive, Peripheral; Neoplasms, Germ Cell and Embryonal; Carcinoma, Transitional Cell; Medulloblastoma; Rhabdomyosarcoma; Hematologic Neoplasms; Ependymoma; Bone metastases; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Rhabdoid Tumor; Hepatoblastoma; Solid tumours; Neoplasms; Wilms Tumor; Glioblastoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Multiple Myeloma; Prostatic Neoplasms, Castration-Resistant; Sarcoma, Ewing; Osteosarcoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Biliary Tract Neoplasms; Solid tumours; Bone Marrow Neoplasms; Carcinoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Central Nervous System Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Sarcoma; Endometrial Neoplasms; Lymphoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Erdafitinib | G-024; JNJ-493; JNJ-42756493; TAR-210; 890E37NHMV | Approved | Astex Pharmaceuticals Inc | Balversa | United States | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Carcinoma, Squamous Cell; Breast Neoplasms; Neuroblastoma; Sarcoma; Prostatic Neoplasms; Bile Duct Neoplasms; Urologic Neoplasms; Histiocytic Sarcoma; Hepatic Insufficiency; Lymphoma; Lymphoma, Non-Hodgkin; Histiocytosis, Langerhans-Cell; Carcinoma, Neuroendocrine; Neoplasms, Neuroepithelial; Glioma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Xanthogranuloma, Juvenile; Neuroectodermal Tumors, Primitive, Peripheral; Neoplasms, Germ Cell and Embryonal; Carcinoma, Transitional Cell; Medulloblastoma; Rhabdomyosarcoma; Hematologic Neoplasms; Ependymoma; Bone metastases; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Rhabdoid Tumor; Hepatoblastoma; Solid tumours; Neoplasms; Wilms Tumor; Glioblastoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Multiple Myeloma; Prostatic Neoplasms, Castration-Resistant; Sarcoma, Ewing; Osteosarcoma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Rogaratinib | BAY-1163877 | Phase 3 Clinical | Bayer AG | Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| SC-0011 | SC-0011 | Phase 3 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| Fisogatinib | BLU-554; CS-3008 | Phase 2 Clinical | Blueprint Medicines Corp | Carcinoma, Hepatocellular | Details |
| EVER-4010001 | EVER-4010001 | Phase 2 Clinical | EverNov Medicines (Zhuhai Hengqin) Co Ltd | Solid tumours | Details |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Roblitinib | FGF-401; NVP-FGF401 | Phase 2 Clinical | Novartis Pharma Ag | Solid tumours; Neoplasms; Carcinoma, Hepatocellular | Details |
| Irpagratinib | ABSK-011 | Phase 2 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours; Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| BPI-43487 | BPI-43487 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Biliary Tract Neoplasms; Solid tumours; Carcinoma, Hepatocellular | Details |
| HS-236 | HS-236 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Solid tumours | Details |
| HS-10340 | HS-10340 | Phase 1 Clinical | Changzhou Hengbang Pharmaceutical Co Ltd, Shanghai Hansoh Biomedical Co Ltd, Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours | Details |
| ICP-105 | ICP-105 | Phase 1 Clinical | Nanjing Tianyin Jianhua Pharmaceutical Technology Co Ltd, Beijing Tiancheng Pharmaceutical Technology Co Ltd, Beijing Innocare Pharma Tech Co Ltd | Liver Neoplasms; Solid tumours | Details |
| ZSP-1241 | ZSP-1241 | Phase 1 Clinical | Guangdong Zhongsheng Pharmaceutical Co Ltd, Wuxi Apptec Co Ltd | Biliary Tract Neoplasms; Liver Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| H3B-6527 | H3B-6527 | Phase 1 Clinical | H3 Biomedicine Inc | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| SYHX-2005 | SYHX-2005; SYHX2005 | Phase 1 Clinical | Cspc Ouyi Pharmaceutical Co Ltd | Solid tumours | Details |
| JAB-6343 | Phase 1 Clinical | Jacobio Pharmaceuticals Co Ltd | Solid tumours | Details | |
| SY-4798 | Phase 1 Clinical | Shouyao Holding (Beijing) Co Ltd | Solid tumours | Details | |
| ABSK-012 | ABSK-012; ABSK012 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| LY-2874455 | LY-2874455 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Leukemia, Myeloid, Acute | Details |
| Rogaratinib | BAY-1163877 | Phase 3 Clinical | Bayer AG | Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| SC-0011 | SC-0011 | Phase 3 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| Fisogatinib | BLU-554; CS-3008 | Phase 2 Clinical | Blueprint Medicines Corp | Carcinoma, Hepatocellular | Details |
| EVER-4010001 | EVER-4010001 | Phase 2 Clinical | EverNov Medicines (Zhuhai Hengqin) Co Ltd | Solid tumours | Details |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Roblitinib | FGF-401; NVP-FGF401 | Phase 2 Clinical | Novartis Pharma Ag | Solid tumours; Neoplasms; Carcinoma, Hepatocellular | Details |
| Irpagratinib | ABSK-011 | Phase 2 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours; Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| BPI-43487 | BPI-43487 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Biliary Tract Neoplasms; Solid tumours; Carcinoma, Hepatocellular | Details |
| HS-236 | HS-236 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Solid tumours | Details |
| HS-10340 | HS-10340 | Phase 1 Clinical | Changzhou Hengbang Pharmaceutical Co Ltd, Shanghai Hansoh Biomedical Co Ltd, Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours | Details |
| ICP-105 | ICP-105 | Phase 1 Clinical | Nanjing Tianyin Jianhua Pharmaceutical Technology Co Ltd, Beijing Tiancheng Pharmaceutical Technology Co Ltd, Beijing Innocare Pharma Tech Co Ltd | Liver Neoplasms; Solid tumours | Details |
| ZSP-1241 | ZSP-1241 | Phase 1 Clinical | Guangdong Zhongsheng Pharmaceutical Co Ltd, Wuxi Apptec Co Ltd | Biliary Tract Neoplasms; Liver Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| H3B-6527 | H3B-6527 | Phase 1 Clinical | H3 Biomedicine Inc | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| SYHX-2005 | SYHX-2005; SYHX2005 | Phase 1 Clinical | Cspc Ouyi Pharmaceutical Co Ltd | Solid tumours | Details |
| JAB-6343 | Phase 1 Clinical | Jacobio Pharmaceuticals Co Ltd | Solid tumours | Details | |
| SY-4798 | Phase 1 Clinical | Shouyao Holding (Beijing) Co Ltd | Solid tumours | Details | |
| ABSK-012 | ABSK-012; ABSK012 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| LY-2874455 | LY-2874455 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms; Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.
