 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| IL3-M82Q7 | Mouse | Biotinylated Mouse IL-33 Protein, His,Avitag™ (MALS verified) |  |
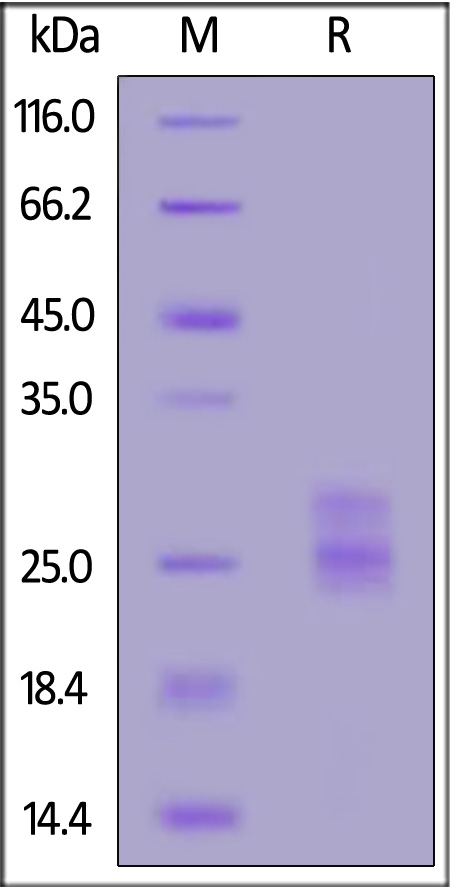
|
|
| IL3-H82H5 | Human | Biotinylated Human IL-33 Protein, His,Avitag™ (MALS verified) |  |

|
|
| IL3-M5245 | Mouse | Mouse IL-33 Protein, His Tag |  |
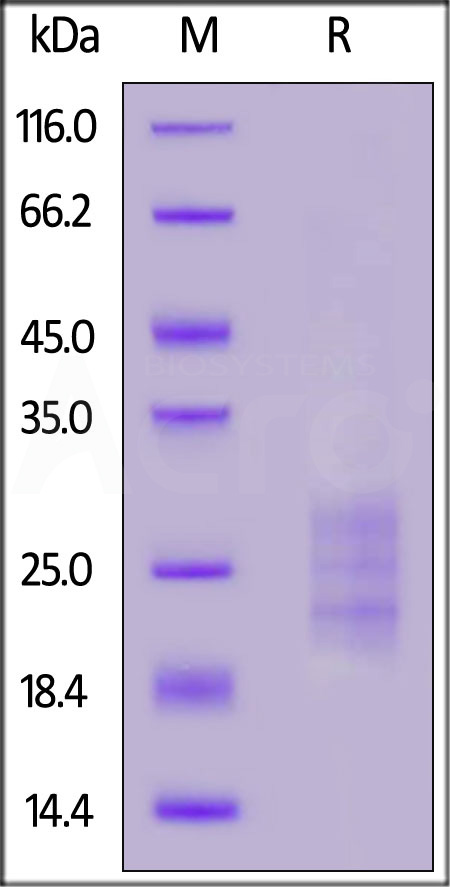
|
|
| IL3-C5245 | Cynomolgus | Cynomolgus IL-33 Protein, His Tag (MALS verified) |  |

|
|
| IL3-H52H7 | Human | Human IL-33 Protein, His Tag (HPLC verified) |  |
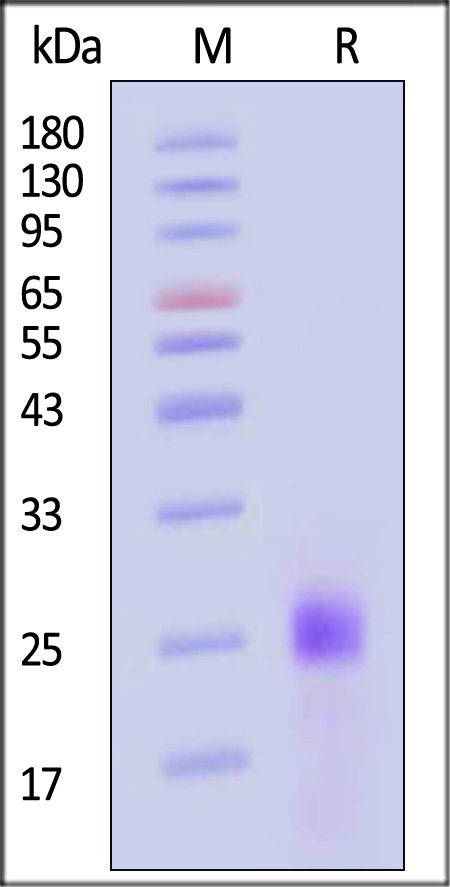
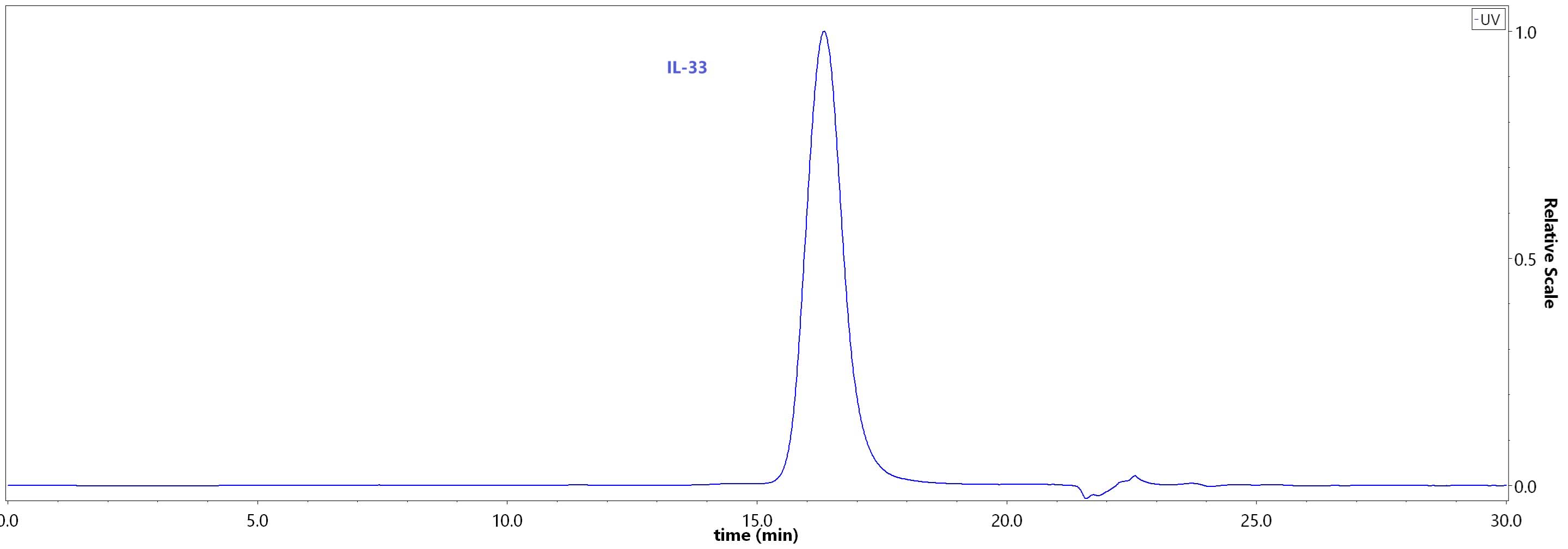
|
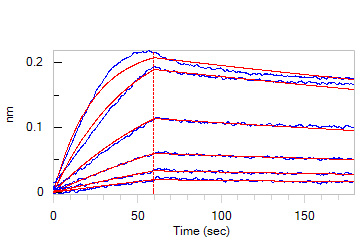
Loaded Human IL-1RL1, Fc Tag (Cat. No. IL1-H5250) on Protein A Biosensor, can bind with Biotinylated Human IL-33, His,Avitag (Cat. No. IL3-H82H5) an affinity constant of 1.81 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Tozorakimab | MEDI-3506 | Phase 3 Clinical | Medimmune | Pneumonia, Viral; Diabetic Nephropathies; Asthma; Pulmonary Disease, Chronic Obstructive; Bronchitis, Chronic; Dermatitis, Atopic | Details |
| Itepekimab | REGN-3500; SAR-440340 | Phase 3 Clinical | Sanofi | Bronchiectasis; Asthma; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| Etokimab | ANB-020 | Phase 2 Clinical | Anaptysbio Inc | Pulmonary Eosinophilia; Asthma; Sinusitis; Peanut Hypersensitivity; Hypersensitivity; Dermatitis, Atopic | Details |
| MT-2990 | MT-2990 | Phase 2 Clinical | Mitsubishi Tanabe Pharma Corp | Pain; Rhinitis, Allergic, Seasonal; Vasculitis; Endometriosis | Details |
| PF-07264660 | PF-07264660 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| PF-06817024 | PF-06817024 | Phase 1 Clinical | Pfizer Inc | Nose Diseases; Dermatitis, Atopic | Details |
| SSGJ-621 | SSGJ-621; 621 | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| CAN-10 | CAN-10 | Phase 1 Clinical | Lund University Foundation | Scleroderma, Systemic; Psoriasis; Myocarditis; Plaque psoriasis | Details |
| Torudokimab | LY-3375880 | Phase 1 Clinical | Eli Lilly And Company | Dermatitis, Atopic | Details |
| GSK-3862995 | GSK-3862995; GSK3862995; GSK3862995B; GSK-3862995B | Phase 1 Clinical | Glaxosmithkline Plc | Pulmonary Disease, Chronic Obstructive | Details |
| QX-007N | QX-007N | Phase 1 Clinical | Qyuns Therapeutics Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| Tozorakimab | MEDI-3506 | Phase 3 Clinical | Medimmune | Pneumonia, Viral; Diabetic Nephropathies; Asthma; Pulmonary Disease, Chronic Obstructive; Bronchitis, Chronic; Dermatitis, Atopic | Details |
| Itepekimab | REGN-3500; SAR-440340 | Phase 3 Clinical | Sanofi | Bronchiectasis; Asthma; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| Etokimab | ANB-020 | Phase 2 Clinical | Anaptysbio Inc | Pulmonary Eosinophilia; Asthma; Sinusitis; Peanut Hypersensitivity; Hypersensitivity; Dermatitis, Atopic | Details |
| MT-2990 | MT-2990 | Phase 2 Clinical | Mitsubishi Tanabe Pharma Corp | Pain; Rhinitis, Allergic, Seasonal; Vasculitis; Endometriosis | Details |
| PF-07264660 | PF-07264660 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| PF-06817024 | PF-06817024 | Phase 1 Clinical | Pfizer Inc | Nose Diseases; Dermatitis, Atopic | Details |
| SSGJ-621 | SSGJ-621; 621 | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| CAN-10 | CAN-10 | Phase 1 Clinical | Lund University Foundation | Scleroderma, Systemic; Psoriasis; Myocarditis; Plaque psoriasis | Details |
| Torudokimab | LY-3375880 | Phase 1 Clinical | Eli Lilly And Company | Dermatitis, Atopic | Details |
| GSK-3862995 | GSK-3862995; GSK3862995; GSK3862995B; GSK-3862995B | Phase 1 Clinical | Glaxosmithkline Plc | Pulmonary Disease, Chronic Obstructive | Details |
| QX-007N | QX-007N | Phase 1 Clinical | Qyuns Therapeutics Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
This web search service is supported by Google Inc.
